Furaneol: Biosynthesis and Pharmacological Activities
General Description
Furaneol, a key flavor compound in fermented soy sauce, is synthesized by yeast and bacteria through complex biological and chemical pathways. Yeast strain Zygosaccharomyces rouxii plays a vital role in its formation, influenced by factors like carbon sources and enzymatic processes. Bacterial synthesis, as seen in Pichia capsulata and Lactococcus lactis subsp. cremoris, highlights the diversity of organisms capable of producing Furaneol. Pharmacologically, Furaneol exhibits antimicrobial properties against pathogens and fungi, without harming human cells. Its ability to activate odor receptors, like OR5M3, sheds light on its sensory perception and potential applications in food science and medicine.Furaneol, a key flavor compound in fermented soy sauce, is synthesized by yeast and bacteria through complex biological and chemical pathways. Yeast strain Zygosaccharomyces rouxii plays a vital role in its formation, influenced by factors like carbon sources and enzymatic processes. Bacterial synthesis, as seen in Pichia capsulata and Lactococcus lactis subsp. cremoris, highlights the diversity of organisms capable of producing Furaneol. Pharmacologically, Furaneol exhibits antimicrobial properties against pathogens and fungi, without harming human cells. Its ability to activate odor receptors, like OR5M3, sheds light on its sensory perception and potential applications in food science and medicine.

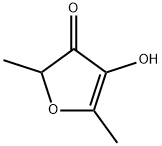
Figure 1. Furaneol
Biosynthesis
Yeast synthesis
The formation of Furaneol(HDMF) by Z. rouxii from D-fructose-1,6-diphosphate was studied under various culture conditions. Growth of Z. rouxii and formation of HDMF was not observed when D-fructose-1,6-diphosphate served as sole carbon source. Although Z. rouxii cells grew in media containing D-glucose as the sole carbon source, HDMF was only produced when D-fructose-1,6-diphosphate was added. The HDMF levels always correlated with the yeast cell count and D-fructose-1,6-diphosphate concentration. Only single labeled Furaneol was formed after addition of 1-13C-D-fructose-1,6-diphosphate but unlabeled furanone was formed in the presence of 13C6-D-glucose. Thus, the carbons of HDMF originate exclusively from exogenously supplied D-fructose-1,6-diphosphate. Higher pH values of the medium had a positive effect on HDMF formation but retarded cell growth resulting in an optimal pH value of 5.1. Salt stress stimulated HDMF production. Addition of o-phenylenediamine, a trapping reagent for α-dicarbonyl (Maillard) intermediates, to the culture medium revealed the formation of three quinoxaline derivatives derived from D-fructose-1,6-diphosphate. Identification of the structures demonstrated for the first time the chemical formation of 1-deoxy-2,3-hexodiulose-6-phosphate, a generally expected but never identified intermediate in the formation pathway of HDMF. Additional enzymatic steps were assumed since HDMF was detected only in the presence of Z. rouxii cells. HDMF is also chemically formed in solutions containing D-fructose-1,6-diphosphate and NAD(P)H at ambient temperature. The NAD(P)H was mandatory and application of labeled precursors indicated a hydride transfer to C-5 or C-6 of the D-fructose-1,6-diphosphate skeleton. It seems that the biological and chemical formation of HDMF from D-fructose-1,6-diphosphate follow similar pathways.1
Bacterial synthesis
Furaneol was detected after 4 days of growth of Pichia capsulata on casein peptone culture medium containing L-rhamnose. Stable isotope ratio mass spectrometry analysis confirmed L-rhamnose as carbon source for HDMF. Time course experiments led to the hypothesis that HDMF is formed by P. capsulata from an intermediate which was generated during thermal sterilization of the culture medium as was proposed for yeast. Similarly, HDMF was detected in media prepared with heated sugar and amino acids as a result of the Maillard reaction. However, enhanced level of HDMF was observed in the same media fermented by Lactococcus lactis subsp. cremoris.1
Receptors
Furanones formed during the Maillard reaction often are natural aroma-determining compounds found in numerous foods. Prominent economically relevant representatives are the structural homologues Furaneol and sotolone, which are important natural flavoring compounds because of their distinct caramel- and seasoning-like odor qualities. These, however, cannot be predicted by the odorants’ molecular shape, rather their receptors’ activation parameters help to decipher the encoding of odor quality. Here, the distinct odor qualities of Furaneol and sotolone suggested an activation of at least two out of our ca. 400 different odorant receptor types, which are the molecular biosensors of our chemical sense of olfaction. While an odorant receptor has been identified for sotolone, a receptor specific for Furaneol has been elusive. Using a bidirectional screening approach employing 616 receptor variants and 187 key food odorants in a HEK-293 cell-based luminescence assay, we newly identified OR5M3 as a receptor specifically activated by Furaneol and homofuraneol.2
References:
[1] SCHWAB W. Natural 4-hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol?).[J]. ACS Applied Energy Materials, 2013. DOI:10.3390/molecules18066936.[2] FRANZISKA HAAG D K Sandra Hoffmann. Key Food Furanones Furaneol and Sotolone Specifically Activate Distinct Odorant Receptors[J]. Journal of Agricultural and Food Chemistry, 2021, 69 37: 10761-11150. DOI:10.1021/acs.jafc.1c03314.
You may like
Related articles And Qustion
Lastest Price from Furaneol manufacturers

US $10.00/ASSAYS2025-08-29
- CAS:
- 3658-77-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton
US $0.00-0.00/KG2025-06-27
- CAS:
- 3658-77-3
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS