Fluxapyroxad: Synthesis and Introduction
Synthesis of Fluxapyroxad
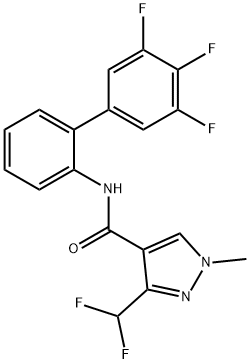
A preparation of fluxapyroxad is likely to involve a metal catalyzed cross coupling reaction to produce the key aniline intermediate 8. The specific synthesis steps are as follows:
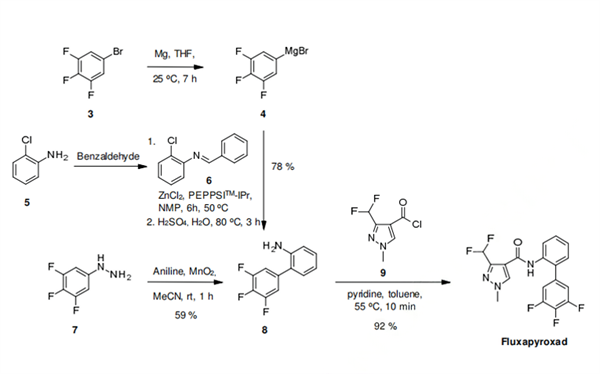
A preparation of fluxapyroxad is likely to involve a metal catalyzed cross coupling reaction to produce the key aniline intermediate 8. Such reactions have precedence in the agrochemical community as demonstrated by both the manufacturing of boscalid's 2-(4- chlorophenyl)aniline which involves the world's largest production volume palladium-catalyzed Suzuki-Miyaura coupling, and the preparation of the 3',4'-dichloro-5-fluorobiphenyl-2- amine of bixafen (2) via a Goossen-type Pd/Cu-catalyzed decarboxylative cross-coupling. In principal, both of those transition metal-catalyzed C-C coupling methods could be applied to the synthesis of fluxapyroxad.
However, the alternative Negishi cross-coupling of a trifluorophenylzinc species generated in situ from its corresponding Grignard 4, with the 2- chloroaniline Schiff base 6, offers an additional approach that seems perfectly suited for the synthesis of fluxapyroxad's 2-(3,4,5,-trifluorophenyl)aniline (8). Additional synthetic methods for the synthesis of aniline 8 could involve a manganese dioxide mediated Gomberg-Bachmann-type regioselective radical arylation of aniline with 3,4,5- trifluorophenylhydrazine (7). Whichever route is used, it is apparent that subsequent amidation of 8 with 3-difluoromethyl-1-methylpyrazole-4-carbonyl chloride (9) will yield fluxapyroxad.
Introduction of Fluxapyroxad
Fluxapyroxad was presented to the public by BASF in 2010. This active ingredient belongs, like the other new fungicides benzovindiflupyr (II), isofetamid (III) and pyraziflumid (IV), to the class of succinate dehydrogenase inhibitors (SDHI), a group of modern and important fungicides blocking the complex II election transport of the respiratory chain. Its biphenylaniline moiety is very similar to the aniline part of two other SDHI, namely boscalid (1) and bixafen (2), and further shares with the latter the 3-difluoromethyl-1- methylpyrazole-4-carboxylic acid motif.
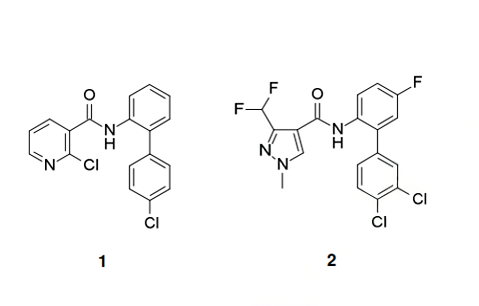
As with many other SDHI, fluxapyroxad can be applied in different crops to prevent a broad range of fungal plant diseases, and is especially efficacious against leaf spot diseases caused by Ascomycetes species.