Flibanserin: A Medication for Hypoactive Sexual Desire Disorder and Female Sexual Interest
General Description
Flibanserin is a medication indicated for individuals with hypoactive sexual desire disorder, characterized by a lack of or reduction in sexual interest or arousal. Although the DSM-5 no longer includes HSDD as a specific diagnosis, it falls under female sexual interest/arousal disorder. Flibanserin acts on serotonin and dopamine receptors, functioning as a 5-HT1A agonist and a 5-HT2A antagonist. It helps restore the balance between excitatory and inhibitory signals in the brain's sexual response circuitry, ultimately improving sexual desire. Clinical trials have demonstrated its efficacy in increasing satisfying sexual events and reducing sexual distress compared to placebo. Discontinuation is recommended if no improvement is seen after 8 weeks.

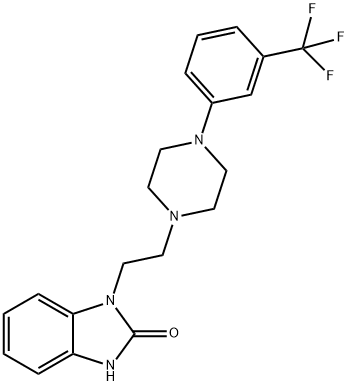
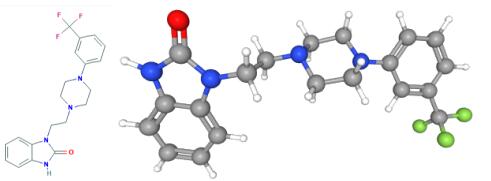
Figure 1. Flibanserin
Treatment indication
Flibanserin, a medication marketed to treat hypoactive sexual desire disorder, is indicated for individuals experiencing significant distress and difficulty related to low sexual desire. HSDD refers to a lack of or reduction in sexual interest or arousal. However, it is important to note that the current diagnostic manual for psychiatry, DSM-5, no longer includes the specific diagnosis of HSDD. In DSM-5, the diagnosis of female sexual interest/arousal disorder combines the previous diagnoses of HSDD and female sexual arousal disorder. FSAID encompasses not only low sexual desire but also an arousal disorder or a combination of both. This diagnostic change was made based on the understanding that sexual response is not always a linear process, and the boundaries between desire and arousal are not clearly defined. According to DSM-5, FSAID is characterized by a lack of or reduction in sexual interest or arousal, including symptoms such as reduced sexual thoughts or fantasies, diminished sexual excitement during sexual activity, decreased initiation of sexual activity, and reduced genital or non-genital sensations during sexual encounters. These symptoms must persist for at least 6 months and cause significant distress to the individual. It is important to rule out other mental disorders, relationship issues, stressors, medical conditions, or the effects of substances or medications as the primary cause of these symptoms. 1
Pharmacological mechanism of action
Flibanserin, a medication used to treat hypoactive sexual desire disorder, exerts its pharmacological mechanism of action through its activity on several serotonin and dopamine receptors. It functions as a serotonin 5-HT1A agonist and a serotonin 5-HT2A antagonist. Additionally, it has some weaker affinity for 5-HT2B, 5-HT2C, and dopamine D4 receptors, acting as an antagonist at those receptors. The binding affinity of flibanserin for 5-HT2B, 5-HT2C, and dopamine D4 receptors is relatively low compared to its affinity for 5-HT1A and 5-HT2A receptors. Moreover, these receptors are not believed to be clinically relevant to the therapeutic efficacy of flibanserin in treating HSDD due to their limited presence in brain regions associated with sexual function. By modulating the activity of 5-HT1A and 5-HT2A receptors, flibanserin is thought to influence the neurocircuitry involved in the sexual response. This helps restore the balance between excitatory and inhibitory signals, ultimately improving sexual desire. Studies conducted in female rats have shown that flibanserin administration increases levels of dopamine and norepinephrine in brain regions associated with the sexual response, such as the prefrontal cortex, nucleus accumbens, and ventral hippocampus. Simultaneously, it decreases serotonin levels in these same regions. Flibanserin is typically prescribed at a dosage of 100 mg once nightly at bedtime. 2
Efficacy
Flibanserin has been shown to be effective in treating premenopausal women with HSDD in three 24-week, randomized, double-blind, placebo-controlled trials. The studies used subjective measures of sexual desire, including the number of satisfying sexual events the Female Sexual Function Index desire domainscore, and the Female Sexual Distress Scale-Revised score. Statistically significant improvement over placebo was seen for the number of monthly SSEs, the FSFI-D score, and the FSDS-R13 score. When responders were separated from nonresponders based on the Patient's Global Impression of Improvement, the overall percentage of responders was greater in individuals taking flibanserin compared to placebo for all three endpoints. Discontinuation of flibanserin is recommended if no improvement is seen after 8 weeks. 2
Reference
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association. 2013.
2. Shapiro D, Stevens D, Stahl SM. Flibanserin - the female Viagra?. Int J Psychiatry Clin Pract. 2017;21(4):259-265.
Related articles And Qustion
Lastest Price from Flibanserin manufacturers

US $20.00/box2025-12-02
- CAS:
- 167933-07-5
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock

US $0.00/kg2025-11-12
- CAS:
- 167933-07-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS