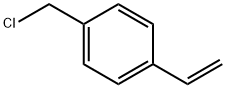
Exploring the Versatility and Applications of 4-Vinylbenzyl Chloride in Modern Chemistry
Introduction
4-Vinylbenzyl chloride is an organochlorine compound with significant importance in various chemical industries. This compound is a derivative of benzyl chloride where the benzene ring is substituted with a vinyl group in the para position. Known for its reactivity, 4-Vinylbenzyl chloride serves as a key intermediate in the synthesis of a variety of polymeric materials and specialty chemicals.

Figure 1 Characteristics of 4-vinylbenzyl chloride
Properties
Physical and Chemical Properties
4-Vinylbenzyl chloride (C9H9Cl) has a molecular weight of 152.62 g/mol. It appears as a colorless to pale yellow liquid with a distinctive aromatic odor. The compound has a boiling point of approximately 195°C and a melting point of -16°C. Its density is about 1.06 g/cm³ at 25°C, and it has a refractive index of 1.553.
Chemically, 4-Vinylbenzyl chloride is highly reactive due to the presence of both the vinyl group and the benzyl chloride moiety. It is prone to polymerization and can readily undergo addition reactions, making it a valuable monomer for polymer synthesis. The presence of the chloride group also allows for nucleophilic substitution reactions, further enhancing its utility in chemical synthesis.
Stability and Reactivity
4-Vinylbenzyl chloride is stable under standard conditions but can polymerize spontaneously when exposed to light, heat, or in the presence of peroxides. It reacts vigorously with strong oxidizing agents, and care should be taken to avoid such conditions. The compound is also sensitive to moisture, leading to the formation of hydrochloric acid, which can cause corrosion of storage containers if not properly managed.
Major Components
The primary component of 4-Vinylbenzyl chloride is the vinylbenzyl chloride itself. It is often synthesized through the chloromethylation of styrene, followed by dehydrohalogenation to introduce the vinyl group. This process yields a mixture of isomers, with the para isomer being the most commercially significant due to its more straightforward reactivity profile.
Applications
Polymer Synthesis
One of the principal uses of 4-vinylbenzyl chloride is in the production of polymers. It acts as a monomer in the formation of poly(vinylbenzyl chloride), which can be further functionalized to create ion-exchange resins, specialty polymers, and other advanced materials. These polymers find applications in water treatment, catalysis, and as components in electronic materials.
Chemical Intermediates
4-Vinylbenzyl chloride is an essential intermediate in the synthesis of a variety of chemical compounds. It is used to produce quaternary ammonium salts, which serve as biocides, phase transfer catalysts, and surfactants. The vinyl group allows for copolymerization with other monomers, leading to the creation of block copolymers and graft copolymers with unique properties tailored for specific industrial applications.
Coatings and Adhesives
The reactivity of 4-Vinylbenzyl chloride makes it suitable for use in coatings and adhesives. Its ability to polymerize and form cross-linked networks contributes to the development of robust, durable coatings with excellent adhesion properties. These coatings are employed in the automotive, aerospace, and marine industries, providing protection against corrosion and wear.
Biomedical Applications
In the biomedical field, 4-Vinylbenzyl chloride-derived polymers are utilized in drug delivery systems and medical devices. The functional groups on these polymers can be modified to interact with biological molecules, enabling targeted drug delivery and improved biocompatibility. Research is ongoing to explore further applications of 4-Vinylbenzyl chloride in developing advanced biomaterials.
Storage and Handling
Proper storage and handling of 4-vinylbenzyl chloride are crucial to ensure safety and maintain its stability. The compound should be stored in a cool, dry, and well-ventilated area, away from direct sunlight and sources of ignition. It is recommended to keep 4-Vinylbenzyl chloride in tightly sealed containers made of materials resistant to corrosion, such as stainless steel or glass-lined vessels.
When handling 4-Vinylbenzyl chloride, personal protective equipment (PPE) such as gloves, goggles, and lab coats should be worn to prevent skin and eye contact. Adequate ventilation or exhaust systems should be in place to avoid inhalation of vapors. In case of spills, the affected area should be ventilated, and the spill should be contained and cleaned up using appropriate absorbent materials.
Conclusion
4-Vinylbenzyl chloride is a versatile and reactive compound with wide-ranging applications in polymer synthesis, chemical intermediates, coatings, adhesives, and biomedical fields. Its chemical properties make it a valuable building block in creating advanced materials with tailored functionalities. However, due to its reactivity, proper storage and handling are essential to ensure safety and maintain the compound's integrity. As research and development in the chemical industry continue to evolve, 4-Vinylbenzyl chloride is likely to remain a critical component in various innovative applications.
References:
[1] DEJAN ?TEFANEC P K. 4-Vinylbenzyl chloride based porous spherical polymer supports derived from water-in-oil-in-water emulsions[J]. Reactive & Functional Polymers, 2005, 65 1: 1-194. DOI:10.1016/j.reactfunctpolym.2005.01.007.[2] KAREL JE?áBEK*. Porogenic Solvents Influence on Morphology of 4-Vinylbenzyl Chloride Based PolyHIPEs[J]. Macromolecules, 2008, 41 10: 3385-3738. DOI:10.1021/ma8002104.
Lastest Price from 4-Vinylbenzyl chloride manufacturers

US $79.00-38.00/kg2025-04-21
- CAS:
- 1592-20-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

US $30.00-10.00/KG2025-04-15
- CAS:
- 1592-20-7
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg