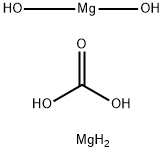
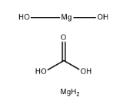
Exploring the Multifaceted World of Magnesium Carbonate Hydroxide: From Chemical Properties to Diverse Applications
Magnesium carbonate hydroxide appears as white, yellowish, grayish-white or brown crystalline solid or crystalline powder. Density: 3-3.1 g cm-3.

Preparation
Magnesium carbonate hydroxide is a white powder formed either by adding an alkaline carbonate (such as sodium carbonate) to a solution of magnesium sulfate or by carbonation of a slurry of magnesium hydroxide followed by boiling of the resulting magnesium carbonate.
Applications
Magnesium carbonate hydroxide has a wide applications: used for fireproofing, heat insulating, filtrating, and to make effervescent magnesium citrate, mineral waters, pigments, and paper; Also used in tooth powders, face powders, polishing compounds, rubber fillers, antacids, and cathartics; Used as a flavoring agent, anticaking agent, drying agent, formulation aid, humectant, lubricant or release agent, nutritive supplement, pH control agent, and processing aid for foods.
Hazard
Magnesium carbonate hydroxide may cause irritation.
Regulatory restrictions
Magnesium carbonate is a GRAS substance under the provisions of the Code of Federal Regulations as miscellaneous and/or gen eral purpose food additives. Magnesium carbonate is listed as GRAS substances migrating to food from paper and paperboard products used in food packaging.
You may like
Related articles And Qustion
Lastest Price from Magnesium carbonate hydroxide manufacturers

US $1.00-1.00/KG2025-04-21
- CAS:
- 39409-82-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt

US $3.60/kg2025-04-18
- CAS:
- 39409-82-0
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 3000tons/month