Vonoprazan Fumarate: Revolutionizing the Treatment of Acid-Related Gastrointestinal Disorders
Introduction
Vonoprazan Fumarate is a potent potassium-competitive acid blocker (P-CAB) that can be used to treat gastrointestinal diseases such as gastroesophageal reflux. In 2022, the U.S. Food and Drug Administration approved two combination products containing Vonoprazan: Voquezna Triple Pak (Vonoprazan, amoxicillin, and clarithromycin) and Voquezna Dual Pak (Vonoprazan and amoxicillin) for the treatment of Helicobacter pylori (H. pylori) infection in adults.
Vonoprazan Fumarate is usually administered orally, 20 mg once daily for the treatment of gastroduodenal ulcers; 20 mg and 10 mg once daily for the treatment and secondary prevention of reflux esophagitis; 10 mg once daily for the secondary prevention of peptic ulcers caused by low-dose aspirin or nonsteroidal anti-inflammatory drugs; 20 mg twice daily in combination with clarithromycin and amoxicillin for the eradication of H. pylori.[1]

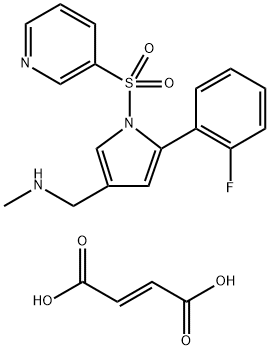
Figure 1 Characteristics ofVonoprazan FuMarate
Mechanism of Action
Vonoprazan Fumarate is a pyrrole derivative with an alkaline pKa of more than 9.0, acid-resistant, highly concentrated in the acidic tubules of gastric parietal cells, and produces an acid-inhibitory effect for more than 24 hours after taking 20 mg. It inhibits H(+), K(+)-ATPase activity in a reversible and potassium-competitive manner and spatially inhibits K+ binding. This blocks K+ activation of H+/K+ ATPase, inhibits the proton pump and prevents gastric acid secretion, thereby reducing gastric acid levels. Its inhibitory potency is approximately 350 times higher than that of the proton pump inhibitor lansoprazole.
Clinical Efficacy
Vonoprazan has multiple advantages over traditional proton pump inhibitors (PPIs), including rapid onset of action, long duration of acid suppression, less inter-individual variability in acid suppression, and minimal effect of diet on its action. These advantages of vonoprazan have been demonstrated in clinical trials conducted for the approval of several acid-related diseases. Most importantly, vonoprazan showed a strong and lasting acid suppression effect from the first day of administration, which was dose-related and further increased within 7 days. A 40 mg dose of vonoprazan almost completely eliminated gastric acid in Japanese and British patients. However, the safety of vonoprazan is also worth discussing, because the use of vonoprazan will inevitably lead to more obvious hypergastrinemia.[2]
References
[1]Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date[J]. Therapeutic advances in gastroenterology, 2018, 11: 1756283X17745776.
[2]Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations[J]. Clinical pharmacokinetics, 2016, 55: 409-418.
Related articles And Qustion
Lastest Price from Vonoprazan FuMarate manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 881681-01-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 900kg

US $0.00-0.00/g2025-04-20
- CAS:
- 881681-01-2
- Min. Order:
- 10g
- Purity:
- 99% HPLC
- Supply Ability:
- 10000