Ethylenediaminetetraacetic Acid Tetrasodium Salt: Versatile Applications in Diverse Fields
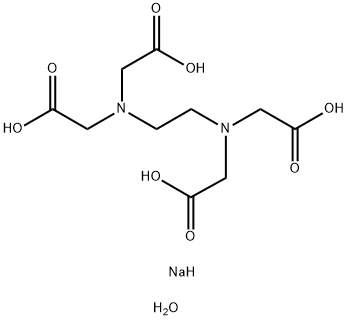
Ethylenediaminetetraacetic acid (EDTA) is a chelator of metal ions. It is a substituted diamine, which has antibacterial activity. Ethylenediaminetetraacetic acid tetrasodium salt (EDTA-4Na) is a small multifunctional organic molecule containing amino and carboxyl groups, which is widely used in analytical chemistry as a complexing agent. It is widely available and inexpensive. Ethylenediaminetetraacetic acid tetrasodium salt is an important complexing agent and metal masking agent. Can be used in the textile industry dyeing, water treatment, color sensitive, pharmaceutical, daily chemical, paper and other industries, as additives, activators, water purification agents, metal ion shielding agent and styrene butadiene rubber industry activator. Dry acrylic industry offset metal interference, improve the color and brightness of the dyed fabric, can also be used in liquid detergent, improve the quality of washing, enhance the washing effect. Ethylenediaminetetraacetic acid tetrasodium salt has been used as a chelating agent to isolate human endometrial stem cell/stromal cells (hEnSCs) from menstrual blood, a chelator in animal and testicular cell isolation, and as a chelator for the culture of neural stem cells.

Ethylenediaminetetraacetic Acid Tetrasodium Salt as a Sacrificing Sodium Ion Source
Recent increasing demands for lithium-ion batteries (LIBs) have led to an escalation in the price of lithium. The limited reserves and uneven distribution of lithium resources must thus be considered in any further development of LIBs. For these reasons, research has recently been directed toward the exploration of alternatives for LIBs. We herein propose the use of a chelating agent, ethylenediaminetetraacetic acid tetrasodium salt (EDTA-4Na), as the sacrificing additive. Although there are 4 mol of sodium per formula unit (EDTA-4Na), the portion of sodium (20%) is lower than that of NaN3 (35%), Na3P (69%), and Na2CO3 (21%) in molar weight. Furthermore, the compound is composed of C–N–O chains; thus, it was assumed that oxidative decomposition may lead to the formation of electroconducting C–N compounds on the surface of the active materials. Moreover, Ethylenediaminetetraacetic acid tetrasodium salt without any π-delocalized groups has been reported to exhibit excellent electron transport property in organic solar cells and act as a strong oxidizing agent.The reaction aggressively developed from 4.2 V, and the resulting anodic current reached approximately 3 mA cm–2, which is related to the dissociation of EDTA-4Na into Na+ and EDTA– ions. This result indicates that a certain amount of Na+ ions was produced from the decomposition of EDTA-4Na salt. Note that the reaction is irreversible with no traceable variation in the current during cathodic sweep to 1.5 V.[1]
For the first time, we investigated the role of organic Ethylenediaminetetraacetic acid tetrasodium salt as a sacrificial salt in a sodium-deficient P2-Na0.67[Al0.05Mn0.95]O2 cathode, which suffers from a small charge capacity because of the insufficient amount of sodium in the structure. The cost-effective EDTA-4Na significantly compensated for the small charge capacity via an electrochemical sacrificing process, which resulted in a sufficient amount of sodium ions and led to the construction of an electroconducting C3N polymeric network. As a result, the formation process of EDTA-4Na/Na0.67[Al0.05Mn0.95]O2//hard carbon full cells was simplified without requiring additional treatment such as presodiation. In addition, the full cells delivered a high discharge capacity of approximately 152 mA h (g-oxide)−1 with a Coulombic efficiency of 89% for the first cycle (formation state), retaining ∼77% of the capacity after 200 cycles. The discovery of Ethylenediaminetetraacetic acid tetrasodium salt as a sacrificing sodium-ion source indicates the substantial possibility of the commercial use of high-capacity sodium-deficient cathode materials for the development of high-energy density SIBs in the near future.
Research into the Impact of Seawater Ionic Composition
Hydraulic fracturing increases production by decontaminating near-wellbore areas and creating artificial fractures to increase the reservoir permeability. The addition of Ethylenediaminetetraacetic acid tetrasodium salt (EDTA-4Na) first chelates the calcium ions in the salt solution and then increases the viscosity of cationic guar gum by electrostatic action. SEM cryo-electron microscope revealed that the fracturing fluid network became denser after the addition of EDTA-4Na, although the viscosification effects varied among different ions. The presence of high-valent anions in Ethylenediaminetetraacetic acid tetrasodium salt interacts electrostatically with the cationic HPG, leading to secondary cross-linking. This increased cross-linking density enhances the intermolecular force within the fluid, making it more challenging for free water to escape. Due to the high content of Na+ and Cl– in seawater and the impactful role of Ca2+ on fracturing fluid performance, this study aimed to investigate how different ions and their concentrations influence the tackifying effect of Ethylenediaminetetraacetic acid tetrasodium salt.[2]
Conversely, when 0.3% EDTA-4Na was added to the HPG fracturing fluid prepared with 1, 3, and 5% calcium chloride solution, the viscosity of the fracturing fluid increased by 4.4, 3.3, and 1.2 mPa·s, respectively. This suggests a diminishing tackifying effect of Ethylenediaminetetraacetic acid tetrasodium salt with increasing salt concentrations. According to the analysis of experimental data, the tackifying effect of EDTA-4Na gradually decreased with the increase in salt concentration. EDTA-4Na, as a chelating agent, can effectively shield Ca2+ and other metal ions in water, reducing their detrimental impact on the fracturing fluid properties. Various metal ions affect the performance of fracturing fluid, among which Ca2+ has the greatest influence on the performance of fracturing fluid. However, data suggest that Ca2+ does not significantly alter the tackifying effect of Ethylenediaminetetraacetic acid tetrasodium salt in the HPG fracturing fluid prepared with calcium chloride solution. Combined with the above experiments, experiments were conducted by using the control variable method to explore the chelating and tackifying ranges of Ethylenediaminetetraacetic acid tetrasodium salt.
Tetrasodium EDTA for the prevention of urinary catheter infections
The aim of this study was to investigate the antimicrobial and anti-encrustation activities of Ethylenediaminetetraacetic acid tetrasodium salt (tEDTA) to evaluate its potential efficacy in preventing CAUTIs and catheter blockages. Herein we investigate the antimicrobial and anti-encrustation activities of tEDTA against uropathogens to evaluate the dual efficacy of this agent for combatting CAUTIs and associated catheter blockages. The antimicrobial activity of tEDTA against uropathogens was assessed using time kill assays performed in artificial urine (AU). The effect of Ethylenediaminetetraacetic acid tetrasodium salt on struvite nucleation and growth, and subsequent catheter encrustation and blockage, was examined in crystallisation studies and in vitro bladder model assays, respectively. These assays were performed in broth media as per CLSI standards; however, tEDTA displays strong affinity to chelate divalent ions present in urine, with chelation of Ca2+ or Mg2+ in a 1 : 1 molar ratio.[3]
The lack of bactericidal activity observed with 7.63 mM tEDTA was due to the absence of sufficient free Ethylenediaminetetraacetic acid tetrasodium salt molecules to interact with the bacteria and exert an antimicrobial effect. While the higher concentration of 28 mM tEDTA provided excess molecules for bacterial interaction, this was not sufficient for bactericidal activity against the challenge uropathogens, as defined by a ≥3 log10 reduction in bacterial density after 24 h exposure to Ethylenediaminetetraacetic acid tetrasodium salt relative to the starting inoculum. In this study we have shown that tEDTA exerts a bacteriostatic effect against P. mirabilis, a particularly problematic pathogen in the pathogenesis of catheter encrustation, as well as preventing precipitation of urinary salts. Struvite crystallisation assays confirmed the mechanism by which Ethylenediaminetetraacetic acid tetrasodium salt impedes struvite growth is via chelation of Mg2+ in solution, with tEDTA concentrations equal to or greater than the Mg2+ required to prevent struvite formation.
References
[1]Jo, Jae Hyeon et al. “New Insight into Ethylenediaminetetraacetic Acid Tetrasodium Salt as a Sacrificing Sodium Ion Source for Sodium-Deficient Cathode Materials for Full Cells.” ACS applied materials & interfaces vol. 11,6 (2019): 5957-5965. doi:10.1021/acsami.8b18488
[2]Wang Y, Li L, Feng Y, Guo L, Zhang Y, Cui Z. Research into the Impact of Seawater Ionic Composition on the Rheological Characteristics of a Cationic Guar Gum-Based Fracturing Fluid. ACS Omega. 2024 Dec 20;10(1):929-939. doi: 10.1021/acsomega.4c07989. PMID: 39829497; PMCID: PMC11740251.
[3]Moore JV, Kim D, Irwin NJ, Rimer JD, McCoy CP. Tetrasodium EDTA for the prevention of urinary catheter infections and blockages. RSC Adv. 2023 Jan 11;13(4):2202-2212. doi: 10.1039/d2ra06418a. PMID: 36741142; PMCID: PMC9832581.
You may like
See also
Lastest Price from Ethylenediaminetetraacetic acid tetrasodium salt manufacturers

US $0.00/KG2025-09-28
- CAS:
- 13235-36-4
- Min. Order:
- 1000KG
- Purity:
- 99.5
- Supply Ability:
- 200TONS/MONTH

US $0.00-0.00/Kg/Bag2025-04-21
- CAS:
- 13235-36-4
- Min. Order:
- 1KG
- Purity:
- 99.0%min
- Supply Ability:
- 20 tons