DL-Limonene: Reactions, biosynthesis and applications
General description
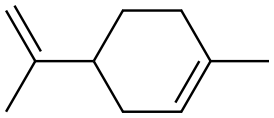
DL-Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the oil of citrus fruit peels. The d-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing.[1] It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common L-isomer is found in mint oils and has a piny, turpentine-like odor.[1] The compound is one of the main volatile monoterpenes found in the resin of conifers, particularly in the Pinaceae, and of orange oil. DL-Limonene is a chiral molecule, and biological sources produce one enantiomer: the principal industrial source, citrus fruit, contains D-limonene ((+)-limonene), which is the (R)-enantiomer. Racemic limonene is known as dipentene. D-Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation. Its appearance is as follows:

Figure 1 Appearance of DL-Limonene.
Reactions
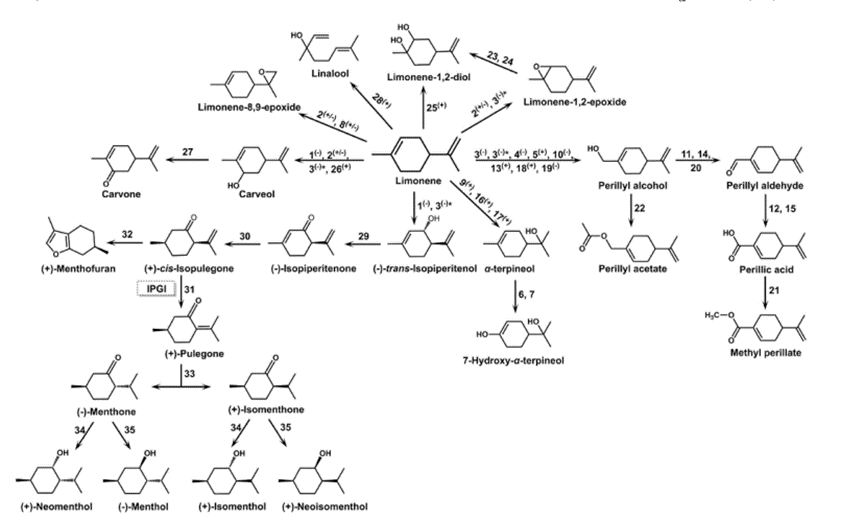
DL-Limonene is a relatively stable monoterpene and can be distilled without decomposition, although at elevated temperatures it cracks to form isoprene.[2] It oxidizes easily in moist air to produce carveol, carvone, and limonene oxide.[1] With sulfur, it undergoes dehydrogenation to p-cymene. DL-Limonene occurs commonly as the d- or (R)-enantiomer, but racemizes to dipentene at 300 °C. When warmed with mineral acid, DL-Limonene isomerizes to the conjugated diene α-terpinene (which can also easily be converted to p-cymene). Evidence for this isomerization includes the formation of Diels–Alder adducts between α-terpinene adducts and maleic anhydride. It is possible to effect reaction at one of the double bonds selectively.Anhydrous hydrogen chloride reacts preferentially at the disubstitutedalkene, whereas epoxidation with mCPBA occurs at the trisubstitutedalkene.
Biosynthesis
In nature, DL-Limonene is formed from geranyl pyrophosphate, via cyclization of a neryl carbocation or its equivalent as shown.[3] The final step involves loss of a proton from the cation to form the alkene. The most widely practiced conversion of DL-Limonene is to carvone. The three-step reaction begins with the regioselective addition of nitrosyl chloride across the trisubstituted double bond. This species is then converted to the oxime with a base, and the hydroxylamine is removed to give the ketone-containing carvone.
Applications
DL-Limonene is common as a dietary supplement and as a fragrance ingredient for cosmetics products.[1] As the main fragrance of citrus peels, DL-Limonene is used in food manufacturing and some medicines, such as a flavoring to mask the bitter taste of alkaloids, and as a fragrance in perfumery, aftershave lotions, bath products, and other personal care products.[1] DL-Limonene is also used as a botanical insecticide.[1] DL-Limonene is used in the organic herbicide "Avenger". It is added to cleaning products, such as hand cleansers to give a lemon or orange fragrance (see orange oil) and for its ability to dissolve oils.[1] In contrast, DL-Limonene has a piny, turpentine-like odor.
DL-Limonene is used as a solvent for cleaning purposes, such as adhesive remover, or the removal of oil from machine parts, as it is produced from a renewable source (citrus essential oil, as a byproduct of orange juice manufacture). It is used as a paint stripper and is also useful as a fragrant alternative to turpentine. DL-Limonene is also used as a solvent in some model airplane glues and as a constituent in some paints. Commercial air fresheners, with air propellants, containing DL-Limonene are used by philatelists to remove self-adhesive postage stamps from envelope paper.[4] DL-Limonene is also used as a solvent for fused filament fabrication based 3D printing. Printers can print the plastic of choice for the model, but erect supports and binders from HIPS, a polystyrene plastic that is easily soluble in limonene. As it is combustible, DL-Limonene has also been considered as a biofuel.
References
[1]d-Limonene. PubChem Compound Database. National Center for Biotechnology Information, US National Library of Medicine. 2017. Retrieved 22 December 2017.
[2]Pakdel, H. (2001). "Production of dl-limonene by vacuum pyrolysis of used tires". Journal of Analytical and Applied Pyrolysis. 57: 91–107.
[3]Mann, J. C.; Hobbs, J. B.; Banthorpe, D. V.; Harborne, J. B. (1994). Natural Products: Their Chemistry and Biological Significance. Harlow, Essex: Longman Scientific & Technical. pp. 308–309.
[4]Butler, Peter (October 2010). "It's Like Magic; Removing Self-Adhesive Stamps from Paper" (PDF). American Philatelist. American Philatelic Society. 124 (10): 910–913.
You may like
Related articles And Qustion
See also
Lastest Price from DL-Limonene manufacturers
US $10.00/KG2025-04-21
- CAS:
- 138-86-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-04-11
- CAS:
- Min. Order:
- 1kg
- Purity:
- 18%
- Supply Ability:
- 20tons