DL-Alanine: Origin and Physiological Roles
General Description
DL-Alanine is a non-essential amino acid that originates from dietary sources, gut microbiota, and endogenous synthesis. It is found in various foods like meats, dairy, and fermented products, where preparation processes can increase its concentration. Gut microbiota significantly contribute to DL-Alanine levels, with specific bacterial species restoring its production in germ-free models. Furthermore, mammals may produce DL-Alanine intrinsically, as evidenced by its presence in saliva. Physiologically, DL-Alanine acts as a co-agonist at N-methyl-D-aspartate receptors, influencing synaptic plasticity and insulin secretion, thereby playing a crucial role in neurological function and glucose metabolism, which has implications for health and disease management.

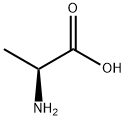
Figure 1. DL-Alanine
Origin
Dietary Origin
DL-Alanine, a non-essential amino acid, is commonly found in a variety of dietary sources that contribute significantly to its presence in mammals, including humans and rodents. This amino acid can be sourced from both natural occurrence in foods and the processes used to prepare them. Various food items, such as fruits and vegetables, dairy products, meats, and fermented foods, contain significant amounts of DL-Alanine. For example, fruits and juices typically contain low amounts of DL-Alanine (0.6-3.8%), while fermented products like cheese and yogurt have notably higher concentrations due to microbial activity. In addition, DL-Alanine levels are often markers of bacterial contamination in products like milk and juices, where high levels (greater than 4%) indicate possible microbial growth. Furthermore, the food processing methods used to enhance flavors and shelf-life, including heating and altering pH levels, can result in the racemization of L-alanine into DL-Alanine, thereby increasing its concentration in processed foods. 1
Gut Microbiota
Alongside dietary contributions, gut microbiota play a crucial role in the synthesis of DL-Alanine within host organisms. Studies involving germ-free and gnotobiotic animal models have demonstrated that the absence of gut bacteria significantly decreases the levels of DL-Alanine in the serum, pointing to the importance of microbial activity in generating this amino acid. Research has shown that specific bacterial species when associated with germ-free mice can restore urinary DL-Alanine levels, supporting the concept that gut microbiota contribute to the endogenous production of DL-Alanine. As such, the complex relationship between dietary intake and microbial synthesis is essential for maintaining adequate levels of DL-Alanine in mammals. Notable experiments, including the administration of antibiotics, have further confirmed the influence of different bacterial populations on urinary DL-Alanine excretion, highlighting the symbiotic interactions between hosts and their microbiomes. 1
Endogenous Synthesis
Beyond dietary sources and microbial synthesis, there is evidence suggesting that mammals may also possess the ability to produce DL-Alanine endogenously. While alanine racemase has been identified in various aquatic invertebrates, its presence in vertebrates has been limited, raising questions about the existence of unknown enzymatic pathways for DL-Alanine production in mammals. Studies have noted detectable levels of DL-Alanine in both germ-free and conventional mice, indicating some degree of intrinsic biosynthesis. Furthermore, significant levels of DL-Alanine have been reported in human saliva, suggesting an endogenous source of this amino acid. This production appears to be stable throughout the day, independent of dietary influences, and may involve localized synthesis in salivary glands. As research continues to uncover the complexities of DL-Alanine’s origins, further exploration of how endogenous synthesis and gut microbiota interplay will be essential for fully understanding this amino acid's role in mammalian physiology. 1
Physiological Roles
Neurological Function
DL-Alanine plays a significant physiological role in the function of N-methyl-D-aspartate receptors (NMDARs), an essential class of glutamate-gated ion channels. These receptors are pivotal in modulating synaptic plasticity, which underlies learning and memory processes. DL-Alanine acts as a potent and stereoselective co-agonist at the glycine-binding site of NMDARs, effectively facilitating the receptors' activation. The binding of DL-Alanine is critical, as it competes favorably with other co-agonists like glycine and D-Ser, enhancing the transport of ions when glutamate binds to NMDARs. Research has demonstrated that DL-Alanine exhibits nearly equal potency to that of glycine and D-Ser in activating NMDARs, making it a crucial component of the receptor's functionality. By promoting the opening of this channel, DL-Alanine significantly influences synaptic responses, which can have broader implications for neurological health and disease states. 2
Glucose Metabolism
DL-Alanine's physiological importance extends beyond neurological roles; it is also implicated in glucose metabolism within pancreatic islets. NMDARs, found in beta-cell lines, play a role in regulating insulin secretion in response to glucose levels. In this context, DL-Alanine contributes to the modulation of insulin release by acting on NMDARs. Studies have shown that inhibiting these receptors enhances glucose-stimulated insulin secretion in both mouse and human islets. The therapeutic potential of targeting NMDARs in diabetes management has been explored, as some clinical trials with NMDAR antagonists demonstrated promising effects on serum insulin levels and blood glucose control. The understanding of DL-Alanine's role suggests that it may influence glucose metabolism similarly to D-Ser, with potential implications in managing metabolic disorders such as type 2 diabetes. 2
Implications for Health
The physiological functions of DL-Alanine, particularly in the context of NMDARs and glucose metabolism, raise important considerations for health and disease prevention. Both neurological disorders and metabolic diseases, such as diabetes, are intricately linked to the functioning of NMDARs. Given DL-Alanine's role as a co-agonist of these receptors, it is conceivable that optimizing DL-Alanine levels could influence synaptic efficiency and metabolic responses. Moreover, research findings indicate that D-Ser supplementation can improve metabolic health, hinting that DL-Alanine may offer similar benefits. Investigating the therapeutic applications of DL-Alanine could open new avenues for addressing disorders characterized by impaired synaptic plasticity or glucose regulation, emphasizing the need for further exploration into its role in human physiology. 2
References:
[1] CINDY J. LEE J V S Tian A Qiu. d-Alanine: Distribution, origin, physiological relevance, and implications in disease[J]. Biochimica et biophysica acta. Proteins and proteomics, 2020, 1868 11. DOI:10.1016/j.bbapap.2020.140482.[2] ERIC T TREXLER. International society of sports nutrition position stand: Beta-Alanine.[J]. Journal of the International Society of Sports Nutrition, 2015, 12. DOI:10.1186/s12970-015-0090-y.
See also
Lastest Price from DL-Alanine manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 302-72-7
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 500kgs

US $1.00/KG2025-04-21
- CAS:
- 302-72-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt