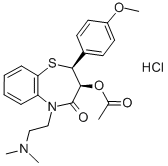
Diltiazem Hydrochloride: Cardiovascular Applications and Liver Safety Profile
Diltiazem hydrochloride is a calcium channel blocker used to prevent chest pain (angina). It may help to increase your ability to exercise and decrease how often you may get angina attacks. This medicine is available in generic form. Diltiazem hydrochloride belongs to a class of medications called calcium-channel blockers. It works by relaxing the blood vessels so the heart does not have to pump as hard. Diltiazem also increases the supply of blood and oxygen to the heart.Diltiazem oral is used in adults alone or in combination with other medicines to treat hypertension (high blood pressure) or symptoms of angina (chest pain). Lowering blood pressure may lower your risk of a stroke or heart attack. Diltiazem injection is used in adults to treat certain heart rhythm disorders such as atrial fibrillation or atrial flutter, or dangerously rapid heartbeats (tachycardia). Get emergency medical help if you have signs of an allergic reaction to diltiazem hydrochloride (hives, difficult breathing, swelling in your face or throat) or a severe skin reaction (fever, sore throat, burning eyes, skin pain, red or purple skin rash with blistering and peeling).

Diltiazem hydrochloride on Drug-Induced Liver Injury
Diltiazem hydrochloride is a first generation calcium channel blocker that is widely used in the therapy of hypertension and angina pectoris. Diltiazem therapy is associated with serum enzyme elevations and has been linked to rare instances of clinically apparent liver injury. Like other calcium channel blockers, diltiazem acts by inhibiting the transmembrane influx of calcium ions into cardiac muscle and vascular smooth muscle cells. The inhibition of calcium flux causes arterial vasodilation and decreases cardiac work and oxygen consumption. Diltiazem hydrochloride, like verapamil (but unlike other calcium channel blockers), also decreases the rate of the sinus node pacemaker and slows atrial-ventricular conduction accounting for its effects on superventricular tachyarrhythmias. Diltiazem was approved in the United States in 1982 and currently several million prescriptions are filled yearly. Current indications for oral forms of diltiazem include hypertension and management of chronic stable angina pectoris, Prinzmetal's or variant angina. Diltiazem hydrochloride is also available in intravenous formulations which are used in therapy of atrial arrhythmias, including atrial fibrillation or flutter and superventricular tachycardia.[1]
Diltiazem hydrochloride therapy is associated with a low rate of mild and transient elevations in serum aminotransferase levels which are usually asymptomatic and often resolve even with continuation of therapy. Clinically apparent, acute liver injury with jaundice due to diltiazem is rare and only isolated case reports have been published. Autoantibody formation has not been described. Thus, liver injury from diltiazem is likely to be idiosyncratic in nature and is typically mild and self-limited with resolution within 4 to 8 weeks of stopping. Acute hepatic injury is listened as a possible adverse event in the diltiazem product label. The mechanism of diltiazem hydrochloride hepatotoxicity is not known, but most cases are probably due to hypersensitivity. Diltiazem is metabolized by the cytochrome P450 system and is an inhibitor of CYP 3A4 activity, which can lead to serious drug-drug interactions and potentiation of the hepatotoxic effects of other medications. Severity of liver injury from Diltiazem hydrochloride ranges from mild and transient serum enzyme elevations to self-limited hepatitis with jaundice. Complete recovery is expected after stopping the drug and recovery is usually rapid (1 to 2 months).
Clinical Experience with Diltiazem in the Treatment of Cardiovascular Diseases
Calcium channel blockers (CCBs) or calcium antagonists reduce the influx of calcium into the cells. Inhibition of calcium channels in the vessels results in vasodilation and, consequently, a lowering of the blood pressure. Some CCBs are also indicated for the treatment of chronic stable angina or atrial arrhythmias, such as diltiazem. The extended-release formulation of diltiazem allows its administration once daily, optimizing antihypertensive and antianginal therapies. Diltiazem hydrochloride belongs to the non-dihydropyridine CCBs. This subclass presents more negative chronotropic and inotropic effects than the dihydropyridine subclass and induces a significant reduction of atrioventricular conduction rate; all of these make non-dihydropyridines useful for acute and chronic treatment as well as for prevention of atrial arrhythmias. A number of studies have analyzed the effects of diltiazem in stable and acute angina. It has been shown that diltiazem hydrochloride increases tolerance to exercise, likely due to its potent dilator effect on coronary arteries.[2]
Several studies have evaluated the antianginal effectiveness of diltiazem hydrochloride alone or in combination with beta-blockers or other CCBs. In a double-blind, randomized, placebo-controlled study, patients were treated four-times daily with 90 mg of diltiazem, 60 mg of propranolol, or a combination of 90 mg of diltiazem and 60 mg of propranolol. Additionally, diltiazem hydrochloride alone was better tolerated than nifedipine alone at maximal effective doses. The combination of the two calcium blockers appeared to be beneficial; however, an increase in side effects was detected. Long-term preventive pharmacotherapy is an alternative approach for some patients. In this regard, some authors have proposed diltiazem as a potential agent for supraventricular tachycardia prophylaxis, after analyzing the effect of diltiazem 270 mg every 8 h in 36 patients. The results demonstrated that 71.9% of the patients returned to normal sinus rhythm after the higher dose of adenosine, whereas the percentage of patients increased to 95% after diltiazem (0.25 mg/kg). Therefore, diltiazem hydrochloride may be a first-choice treatment in patients with narrow-complex supraventricular tachycardia.
Diltiazem Dosing Strategies in the Management of Atrial Fibrillation
Atrial fibrillation (AFIB) is a commonly encountered arrhythmia in the hospital setting and the primary diagnosis for over 600,000 emergency department (ED) visits and 460,000 hospitalizations in the United States, each year. For many ED physicians, Diltiazem hydrochloride has become the preferred agent to achieve rate control of AFIB with rapid ventricular rate (AFIB-RVR). Diltiazem is effective in treating AFIB-RVR by slowing conduction through the atrioventricular node (AV) and by prolonging AV node refractoriness. Recent studies have suggested that low-dose diltiazem dosing strategies may be as effective as standard dosing for rate control in AFIB, thus prompting healthcare providers to prescribe < 0.25 mg/kg diltiazem for AFIV-RVR. In our experience, Diltiazem hydrochloride dosing strategies vary among providers. This study investigated diltiazem dosing strategies administered to patients who present with AFIB-RVR in a suburban ED and compared doses with time to achieve ventricular rate control.[3]
We report the dosing strategy to achieve timely rate control (HR < 100 bpm) without clinically significant hypotension is at least 0.13 mg/kg diltiazem hydrochloride. Future studies need to be done to investigate the association of weight-based diltiazem, HR control and patient length of stay. In our study, patients who presented to the ED with AFIB-RVR achieved successful HR control in less time when treated with weight-based diltiazem bolus ≥ 0.13 mg/kg compared to patients treated with < 0.13 mg/kg. Higher doses of diltiazem hydrochloride were not associated with higher incidence of hypotension or bradycardia. Prospective studies comparing weight-based dosing strategies to non-weight-based diltiazem are warranted. The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional.
References
[1]LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Diltiazem. [Updated 2017 Jan 11].
[2]Rodríguez Padial L, Barón-Esquivias G, Hernández Madrid A, Marzal Martín D, Pallarés-Carratalá V, de la Sierra A. Clinical Experience with Diltiazem in the Treatment of Cardiovascular Diseases. Cardiol Ther. 2016 Jun;5(1):75-82. doi: 10.1007/s40119-016-0059-1. Epub 2016 Mar 25. Erratum in: Cardiol Ther. 2016 Jun;5(1):83-4. doi: 10.1007/s40119-016-0062-6. PMID: 27016085; PMCID: PMC4906086.
[3]Bishop J Jr, Akram G. Diltiazem Dosing Strategies in the Management of Atrial Fibrillation With Rapid Ventricular Rate. Cureus. 2021 Oct 16;13(10):e18829. doi: 10.7759/cureus.18829. PMID: 34804686; PMCID: PMC8592802
See also
Lastest Price from Diltiazem hydrochloride manufacturers
US $1.00-4.00/KG2025-09-11
- CAS:
- 33286-22-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $0.00/KG2025-05-06
- CAS:
- 33286-22-5
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG