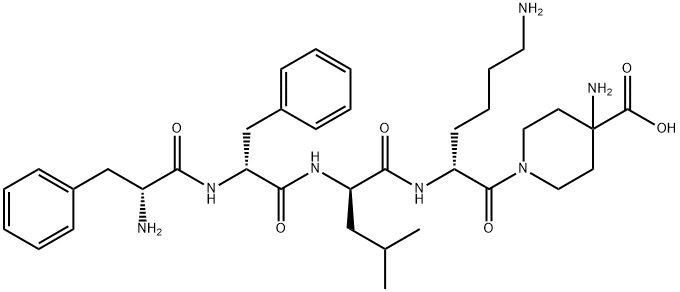
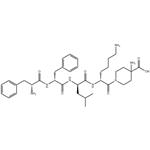
Difelikefalin: Synthesis and Introduction
Synthesis of Difelikefalin
Difelikefalin is synthesised using piperidine amino acid as a raw material by chemical reaction. The specific synthesis steps are as follows:
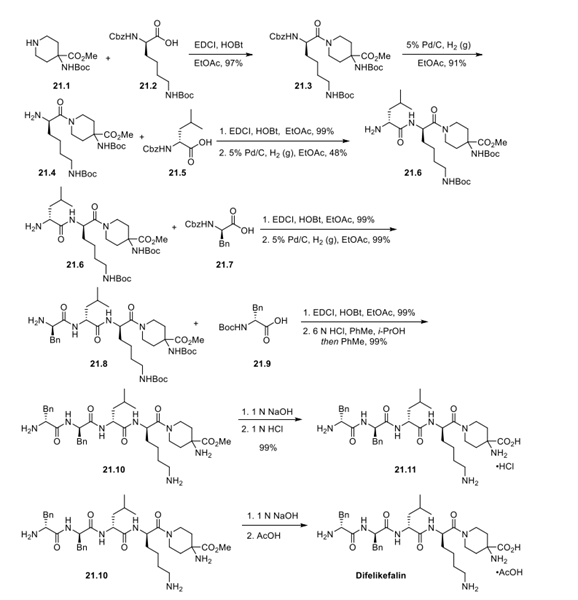
The piperidine amino acid 21.1 was coupled to the fully protected D-lysine analog, 21.2, under standard EDCI/HOBt peptide coupling conditions, providing the fully protected dipeptide 21.3 in 97% yield. Standard hydrogenation conditions resulted in removal of the N-terminal benzyloxycarbonyl protecting group, revealing amine 21.4 in a 91% yield. Dipeptide 21.4 was then treated with protected D-Leu (21.5) and the same peptide coupling procedure followed by Cbz deprotection, affording tripeptide 21.6 in 99% and 48% yield, respectively. It is worth noting that the reduced yield of tripeptide 21.6 is likely a result of the crystallization implemented for purification (using cyclohexane and EtOAc) rather than a lack of reaction progression. Pure tripeptide 21.6 was treated with protected D-Phe and the same peptide coupling procedure followed by Cbz deprotection, affording the tetrapeptide 21.8 in 99% yield for each step.
Treatment of tetrapeptide 21.8 with D-BocPhe under standard peptide coupling conditions provided the fully protected pentapeptide in 99% yield. Global Boc deprotection was accomplished using 6 N HCl in i-PrOH, followed by addition of toluene to the reaction mixture, resulting in precipitation of the pentapeptide methyl ester 21.10 in 99% yield. Next, methyl ester 21.10 was subjected to standard saponification conditions and the reaction quenched with 1 N HCl, providing the fully elaborated pentapeptide 21.11 as an HCl salt. While not disclosed, one could envision quenching the saponification of pentapeptide 21.10 with acetic acid to provide difelikefalin as its marketed acetate salt.
Introduction of Difelikefalin
Difelikefalin was discovered by Cara Therapeutics and developed by Vifor Pharma for the treatment of moderate to severe pruritus (itching). Difelikefalin is a highly selective kappa opioid receptor agonist approved by both the USFDA and EMA in 2021. The fully unnatural pentapeptide is delivered intravenously. The drug is designed to be peripherally restricted, which limits known side effects such as sedation, dysphoria, and hallucinations.
You may like
See also

US $0.00-0.00/KG2025-10-12
- CAS:
- 1024828-77-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500kg
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1