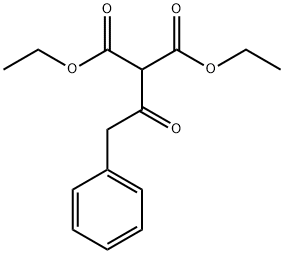
Diethyl(phenylacetyl)malonate: Preparation and Application
Physicochemical property
Diethyl(phenylacetyl)malonate has a boiling point of 120 °C. Its density is predicted to be 1.148±0.06 g/cm3
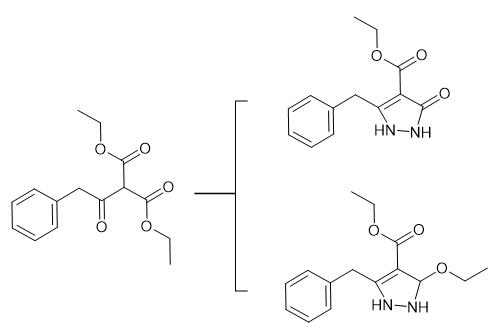
Fig .1 As a synthetic intermediate.
Preparation
The anhydrous ethanol, which was co-steamed with diethyl phthalate and sodium metal, was added to the mixture of diethyl malonate, carbon tetrachloride and magnesium by drops. The reaction was started by heating and the temperature was controlled to smooth the reaction. Then add anhydrous ether, heat for 1h, add the ether solution of benzyl chloride (add slowly, do not make the reaction too intense). After the reaction, the oil layer was separated by cooling and water, and the diethyl ether was steamed under pressure to obtain Diethyl(phenylacetyl)malonate.
Application
As a synthetic intermediate
Diethyl(phenylacetyl)malonate can be used to synthesize methylanhydroacetonebenzil. While sym-dimethylanhydroacetonebenzil when treated with acidic dehydrating agents forms a bimol. compd. which dissocs. in most of its reactions, anhydroacetonebenzil (I) when treated in the same way gives a bimol. rearrangement product which no longer dissocs. A monoalkyl deriv. of I should fall between these 2 extremes. The reactions of such a bimol. product with maleic anhydride (II) and with alc. alkali are studied because they may disclose its structure and the presence of an angular Ph group. When a mixt. of 64 g. Am deriv. of I, 75 cc. CHCl3, 28 cc. Ac2O, and 4 cc. concd. H2SO4 is refluxed for 15 min., 150 cc. MeOH added after standing for 2 h., and the mixt. kept in a refrigerator for 3 days, 62% 3a,4,7,7a-tetrahydro-3,3a,5,6-tetraphenyl-2,7-diamyl-4,7-methanoindene-1,8-dione (III) (IV, R = Am), m. 153°, is obtained. The Am deriv. of I gives a 2,4-dinitrophenylhydrazone, small reddish orange needles, m. 199°. The Me deriv. (V) (IV, R = Me) m. 230° (cf. Japp and Meldrum, J. Chem. Soc. 79, 1024(1901)). III and V show in the Grignard machine the presence of 1 active H and one other addn. Neither III nor V reacts with II in boiling C6H6, indicating that no appreciable dissocn. takes place. When, however, 24 g. V and 10 g. II are heated at 220° until CO is no longer evolved, the solid residue extd. with 125 cc. C6H6 and recrystd. from C6H3Cl3 and from Ac2O, 10 g. 1-methyl-7,8-diphenylbicyclo[2.2.2]-7-octene-2,3,5,6-tetracarboxylic acid dianhydride (VI) (VII, R = Me), m. 325°, b7 340-60°, is obtained. From the C6H6 ext. on addn. of 125 cc. ligroin, 16 g. monoanhydride (C33H24O4), m. 288°, is formed by addn. of 1 mol. II and the loss of CO and C6H6. III and II at 190-200° or on refluxing a mixt. of 10 g. III and 10 g. II in 20 cc. C6H3Cl3 contg. 2 drops H2SO4 give the 1-Am deriv. (VIII) (VII, R = Am), crystals from o-C6H4Cl2, m. 300°. VIII sublimes at 1 mm. The Am monoanhydride, C41H40O4, m. 255°. To det. the structure of VI and VIII the action of alkali is used which cleaves the CO bridge, and because the scission occurs on both sides of the CO group, 2 isomeric acids (IX and X) are formed. When 46 g. VI in 300 cc. abs. EtOH is refluxed with 23 g. KOH for 4 h., 2 l. H2O added, and the mixt. acidified, 20 g. of a mixt. of acids seps. which is filtered and dried. Extn. with AcOH gives 10.1 g. X, m. 145-6° after recrystn. from EtOH. Recrystg. of the residue from a large vol. of AcOH gives IX contg. 1 mol. AcOH, m. 269°. When alc. EtONa is used, the corresponding Et ester, m. 180°, is formed. To det. the structure of IX and X they are oxidized by dropwise addn. of 43 g. KMnO4 in 72 cc. H2O to a vigorously stirred hot soln. of 48 g. IX in 400 cc. H2O contg. 12 g. K2CO3, giving 2,3,6-triphenyl-p-toluic acid (XI), m. 288-9°, and a small amt. of BzOH. X, under the same conditions, loses HCO2H and gives a ketone, C35H28O, m. 164°, which dissolves in concd. H2SO4 with a brilliant red color. When 2.6 g. α-Me deriv. of I (XII), 1 g. II, and 15 cc. AcOH are refluxed for 5 h., the mixt. made alk., treated with Norit, acidified, and extd. with C6H6, 3-methyl-4,5-diphenyl-7-keto-1,2,3,6-tetrahydro-3,6-methanobenzene-1,2-dicarboxylic acid, m. 187-8°, is obtained. When the alk. treatment is omitted, there is obtained the anhydride, C22H16O4.C6H6, sinters at 98° with loss of C6H6 and m. 139-40° (2,4-dinitrophenylhydrazone, m. 218°). If the reaction mixt. is distd. 3-methyl-4,5-diphenylphthalic anhydride (XIII), m. 198°, is formed. XIII is also obtained by heating 5 g. XII at 160-70°, adding 4 g. (≡CCO2H)2, and raising the temp. to 190° for 5 min. When 5 g. XII is heated with PhC≡CCO2H for 50 min. at 160-70°, CO is evolved and 3,4,6-triphenyl-o-toluic acid (XIV), m. 268°, is formed. Me ester, prepd. in a similar way but with distn. of the reaction mixt., b2 300°, m. 152-3° after recrystn. from iso-PrOH. XII and (≡CCO2Me)2 give Me 3-methyl-4,5-diphenylphthalate, long needles, m. 114-15°, which is also obtained by refluxing a mixt. of XIII, MeOH, and H2SO4 for 3 h. Decarboxylation of XI or XV by heating with Cu powder gives 2,3,6-triphenyltoluene, m. 130°, which is also formed when 5 g. XII and CH≡CPh are heated for 5 h. at 160-70° and the residue is distd. at 9 mm. Decarboxylation of VIII, by using a mixt. of the Na salt and CaCO3 (cf. A. and Gates, C.A. 36, 6527.7), and distn. of the reaction mixt., b12 205-20°, gives 2,3-diphenyltoluene, viscous oil, b2 150°. The results prove definitely the presence of an angular Ph group in IX and X and therefore also in V [1].
Diethyl(phenylacetyl)malonate can be used to synthesize a dye. The absorption spectrum and soly. of the pure dye and the quant. method for detn. of II (Ogata and Yamanouchi, C.A. 25, 1861) were examd. The absorption curve obtained with benzene, Et2O, and CHCl3 solns. of the dye are smooth curves with on absorption max. at 560, 570, and 590 mμ, resp. The resp. mol. coeffs. of light absorption are 1.73 × 104, 1.83 × 104, and 1.67 × 104, and solubilities are 0.6 × 10-4, 1.03 × 10-4, and 1.16 × 10-4, mol./l., resp. The purple Et2O soln. obtained by the reaction of I and II by Ogata's method gives a light absorption curve with a max. at 570 mμ, similar to that of the above dye. Since a similar curve is obtained when the reaction is carried out with the addn. of urea, apparently the presence of urea has no appreciable effect. However, the addn. of urea apparently forms a by-product that shows absorption in a wide range in a long wave-length portion. Moreover, at less than 0.5 mg. of II the amt. of the acid and light absorption are not proportional and do not change even when the reaction time is prolonged to allow increased production of the dye. Above 2 mg.II the dye formed does not completely dissolve, so that the results of quant. analysis are irregular. These facts are explained from the observed soly. The prolongation of the reaction time lowers the accuracy of the analysis. The prepn. of phenacetyl malonic ester from malonic ester and phenacetyl chloride gives better results, in a shorter time and with simple procedures by the use of Mg in EtOH as described by Lund (C.A. 28, 5040.3) rather than the condensation by Na in ether heretofore used [2].
References
[1] Nozaki Y. A dye formed by the naphthoresorcinol reaction of glucuronic acid. III [J]. Yakugaku Zasshi, 1944, 64(No. 8A), 14-15.
[2] Allen C F H, VanAllan J. EVIDENCE FOR THE PRESENCE OF AN ANGULAR PHENYL GROUP IN THE BIMOLECULAR PRODUCT FROM METHYL-ANHYDROACETONEBENZIL[J]. The Journal of Organic Chemistry, 1945, 10(4): 333-340.
You may like
Related articles And Qustion
See also
Lastest Price from Diethyl(phenylacetyl)malonate manufacturers

US $0.00-0.00/kg2025-05-12
- CAS:
- 20320-59-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $6.00/kg2025-04-21
- CAS:
- 20320-59-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month