Diethyl(phenyl acetyl)malonate: A Comprehensive Overview
Introduction
Diethyl(phenylacetyl)malonate, a significant compound in organic chemistry, has garnered attention for its versatile applications and unique properties.

Figure 1 Characteristics of Diethyl(phenylacetyl)malonate
Properties
Chemical Structure and Formula
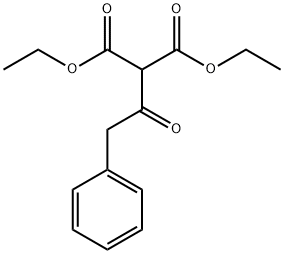
Diethyl(phenylacetyl)malonate is a diester of malonic acid. Its molecular formula is C15H18O5, and it has a molar mass of 278.30 g/mol. The compound features a malonate core with a phenylacetyl group attached to one of the methylene carbons and two ethyl ester groups. The IUPAC name for this compound is Diethyl 2-(2-phenylacetyl)propanedioate.
Physical Properties
This compound is typically found as a crystalline solid with a melting point ranging from 50°C to 55°C. It is sparingly soluble in water but highly soluble in organic solvents such as ethanol, methanol, and dichloromethane. These solubility characteristics make it suitable for various organic synthesis reactions where water solubility might pose a challenge.
Chemical Behavior
Diethyl(phenylacetyl)malonate is known for its reactivity in organic synthesis. The ester groups are prone to hydrolysis under acidic or basic conditions, leading to the formation of malonic acid derivatives. The phenylacetyl group adds an aromatic ring that can participate in electrophilic and nucleophilic aromatic substitution reactions, further enhancing the compound's versatility.
Composition
Molecular Composition
As previously mentioned, the molecular formula of Diethyl(phenylacetyl)malonate is C15H18O5. The structure consists of a central malonate moiety (a 1,3-dicarboxylate ester), an ethyl group attached to each ester, and a phenylacetyl group, which is a benzene ring attached to an acyl group. This combination of functional groups allows for a wide range of chemical reactions, making it a valuable intermediate in synthetic chemistry.
Structural Features
The presence of both ester and ketone functional groups in Diethyl(phenylacetyl)malonate provides multiple reactive sites. The carbonyl groups in the ester moieties can engage in nucleophilic addition reactions, while the phenylacetyl group can stabilize negative charges through resonance, making it an excellent candidate for various organic transformations.
Applications
Pharmaceutical Industry
Diethyl(phenylacetyl)malonate is widely used in the pharmaceutical industry as an intermediate in the synthesis of various therapeutic agents. Its structure allows it to be a precursor in the formation of anti-inflammatory drugs, anticonvulsants, and other bioactive molecules. The compound’s ability to undergo diverse chemical reactions makes it a valuable tool in drug design and development.
Agrochemical Industry
In the agrochemical sector, Diethyl(phenylacetyl)malonate serves as a precursor for the synthesis of herbicides and pesticides. Its chemical properties enable the development of compounds that can effectively target specific biological pathways in pests and weeds, leading to more efficient and environmentally friendly agricultural practices.
Organic Synthesis
Diethyl(phenylacetyl)malonate is a staple in organic synthesis labs. Its versatility allows it to be used in the construction of complex molecules through reactions such as alkylation, acylation, and condensation. Researchers often employ this compound in the synthesis of heterocyclic compounds, which are prevalent in many natural products and pharmaceuticals.
Toxicity
General Toxicity
While Diethyl(phenylacetyl)malonate is a valuable compound in various industries, it is essential to consider its toxicity. Studies have shown that the compound can be harmful if ingested, inhaled, or absorbed through the skin. Acute exposure may irritate the respiratory tract, skin, and eyes. Prolonged exposure could potentially lead to more severe health effects, including damage to internal organs.
Safety Precautions
Handling Diethyl(phenylacetyl)malonate requires proper safety measures to minimize exposure risks. Personal protective equipment (PPE) such as gloves, safety goggles, and lab coats are essential. Working in a well-ventilated area or using a fume hood can help reduce inhalation hazards. In case of contact, immediate washing with plenty of water and seeking medical attention is advised.
Environmental Impact
The environmental impact of Diethyl(phenylacetyl)malonate is also a concern. Improper disposal can lead to contamination of water sources and soil, potentially harming aquatic life and disrupting ecosystems. It is crucial to follow regulatory guidelines for the disposal of chemical waste and to implement measures that prevent accidental release into the environment.
Conclusion
Diethyl(phenylacetyl)malonate stands out as a compound of significant interest in the chemical industry due to its diverse applications and reactive properties. Its role in the pharmaceutical and agrochemical industries, along with its utility in organic synthesis, underscores its importance. However, it is equally important to recognize the potential health and environmental risks associated with its use. By adhering to safety protocols and regulatory guidelines, professionals can safely and effectively utilize Diethyl(phenylacetyl)malonate in their work, continuing to unlock its potential in various scientific and industrial applications.
References:
[1] E R OLIVERA. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon.[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95 11. DOI:10.1073/pnas.95.11.6419.[2] J. LUENGO E R O José L García. The phenylacetyl‐CoA catabolon: a complex catabolic unit with broad biotechnological applications[J]. Molecular Microbiology, 2001, 39 1. DOI:10.1046/j.1365-2958.2001.02344.x.
Related articles And Qustion
Lastest Price from Diethyl(phenylacetyl)malonate manufacturers

US $0.00-0.00/kg2025-05-12
- CAS:
- 20320-59-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $6.00/kg2025-04-21
- CAS:
- 20320-59-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month