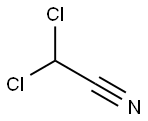
Dichloroacetonitrile: a Nitrogenous disinfection by-products
Introduction
Nitrogenous disinfection by-products (N-DBPs) are frequently found in drinking water, wastewater, and swimming pool water. One of the most toxic and prevalent N-DBPs is haloacetonitriles (HANs), including dichloroacetonitrile (DCAN), the most prominent HAN species. HANs are highly cytotoxic and genotoxic, with toxicity values 1–2 orders of magnitude higher than the regulated 5 haloacetic acids. Their frequent occurrence in drinking water is a concern. The sum of 4 major HANs (i.e. dichloro-, bromochloro-, dibromo- and trichloro-acetonitrile) was reported up to 36 μg/L and at a mean value of 4.0 μg/L in Australia and US, respectively. However, they are not currently regulated worldwide, only World Health Organization (WHO) regulates DCAN and dibromoacetonitrile with guideline values of 20 μg/L and 70 μg/L, respectively.
A study differentiated the contributions of the oxidative and reductive species generated in a VUV system to DCAN degradation.
VUV irradiation at 185 nm degraded DCAN effectively under an oxygen-free condition. eaq–, as a reductive species formed in this process, took a dominant responsibility for DCAN degradation. High reactivity between eaq– and DCAN was found using both QSAR and competition kinetics methods. The reaction rate constant was determined to be 3.16 to 3.76 × 1010 M−1s−1. eaq− was significantly consumed by DO but still played an essential role in DCAN degradation at the natural DO level. The degradation primarily followed a reductive dechlorination pathway initiated by the cleavage of C–Cl bonds by eaq–. Cl− was the major inorganic product of DCAN degradation.
Degradation mechanisms
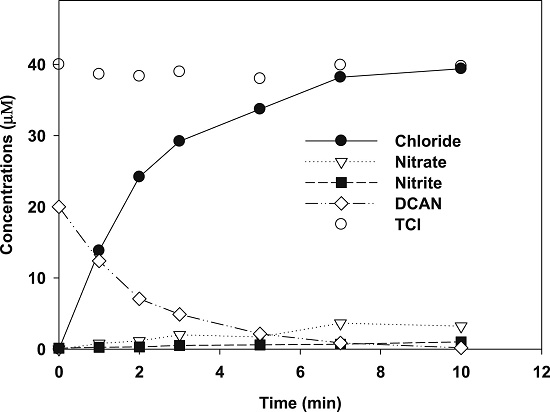
The figure below displays the changes in chloride, nitrite, nitrate and total chlorine (TCl) concentrations during VUV treatment of DCAN under oxygen-free conditions. The major product was chloride ion (Cl−), while much lower quantities of nitrate and nitrite were detected. Cl− accounted for almost 100% of TCl, indicating complete dechlorination of DCAN during the treatment. The nitrogen element was unbalanced, indicating that other organic nitrogen products were formed. The much lower yields of nitrate and nitrite (less than 20% of the amount of degraded DCAN) indicated limited breakage of C≡N triple bonds in DCAN molecules.
To confirm the DCAN degradation mechanisms, the distribution of Fukui indices and the lowest unoccupied molecular orbital (LUMO) of DCAN molecules were calculated. The maximum Fukui index for nucleophilic attack (fmax+) reflecting affinity to nucleophilic attack was located on Cl of the DCAN molecules. This is associated with the LUMO, dominated by C–Cl bonds. Both results suggested that the sites of the DCAN molecules attacked by eaq– were the C–Cl bonds.
Environmental fate
Dichloroacetonitrile and dibromoacetonitrile are very mobile in soil and are expected to leach. In moist, alkaline soils, dichloroacetonitrile and dibromoacetonitrile may hydrolyse. In water, dibromoacetonitrile is lost through hydrolysis, which occurs at a faster rate in alkaline waters and in the presence of chlorine. Roughly 10% and 60% of dichloroacetonitrile and 5% and 20% of dibromoacetonitrile are lost via hydrolysis in 10 days at pH 6 and 8, respectively. Volatilization losses are minimal, and adsorption to sediment and bioconcentration in aquatic organisms is not expected.
Short-term exposure
Dichloroacetonitrile in corn oil was administered to CD rats (10 per sex per dose) by gavage at doses of 0, 12, 23, 45 or 90 mg/kg of body weight per day for 14 days. In males, decreased body weight gain was observed at the three highest doses, whereas decreased weight gain in females was noted only in the highest dose group. Significantly increased levels of serum glutamate–pyruvate transaminase in females in the highest dose group and of alkaline phosphatase levels in the highest dose group in males and the two highest dose groups in females were observed. Relative liver weight was statistically significantly increased in males in all dose groups. The relative liver weights in males were 13%, 26%, 42% and 45% greater than controls at doses of 12, 23, 45 and 90 mg/kg of body weight per day, respectively. In female rats, both relative and absolute liver weights were statistically significantly elevated beginning at 23 mg/kg of body weight per day, with relative liver weights 36%, 40% and 31% greater than controls at 23, 45 and 90 mg/kg of body weight per day. No consistent compound-related effects were observed in any of the measured haematological, serum chemistry or urinary parameters. The LOAEL for this study was identified as 12 mg/kg of body weight per day, the lowest dose tested, based on increased relative liver weight in males.
[1] Huang Huang. “Dichloroacetonitrile and Dichloroacetamide Can Form Independently during Chlorination and Chloramination of Drinking Waters, Model Organic Matters, and Wastewater Effluents.” 环境科学与技术 46 19 (2012): 10624–10631.
[2] Microsoft Word - Halogenated Acetonitriles.doc https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/halogenated-acetonitriles-bd.pdf?sfvrsn=2c7c517e_4
References:
[1] HUANG HUANG. Dichloroacetonitrile and Dichloroacetamide Can Form Independently during Chlorination and Chloramination of Drinking Waters, Model Organic Matters, and Wastewater Effluents[J]. 环境科学与技术, 2012, 46 19: 10383-10860. DOI:10.1021/es3025808.[2] Halogenated acetonitriles.[J]. IARC monographs on the evaluation of carcinogenic risks to humans, 1991, 52.
You may like
Lastest Price from Dichloroacetonitrile manufacturers

US $7.00/kg2019-07-06
- CAS:
- 3018-12-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg