Dibutyl phthalate: Degratation, applications and toxicity
General description
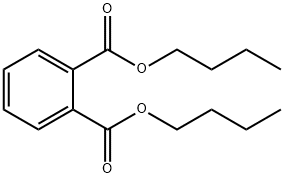
Dibutyl phthalate (DBP) is an organic compound which is commonly used as a plasticizer because of its low toxicity and wide liquid range. Dibutyl phthalate is produced by the reaction of n-butanol with phthalic anhydride. Dibutyl phthalate is an important plasticizer that enhances the utility of some major engineering plastics, such as PVC. Such modified PVC is widely used in plumbing for carrying sewerage and other corrosive materials. Its appearance is as follows:

Figure 1 Appearance of Dibutyl phthalate
Degradation
Hydrolysis of Dibutyl phthalate leads to phthalic acid and 1-butanol.[1] Monobutyl phthalate (MBP) is its major metabolite. Biodegradation by microorganisms represents one route for remediation of DBP. For example, Enterobacter species can biodegrade municipal solid waste—where the DBP concentration can be observed at 1500 ppm—with a half-life of 2–3 hours. In contrast, the same species can break down 100% of dimethyl phthalate after a span of six days. The white rot fungus Polyporus brumalis degrades Dibutyl phthalate. Dibutyl phthalate is leached from landfills. [2] Dibutyl phthalate has a low vapor pressure of 2.67 × 10−3 Pa. Thus Dibutyl phthalate does not evaporate readily (hence its utility as a plasticizer). The Henry's Law constant is 8.83 × 10−7 atm-m3/mol. [1]
Applications
Dibutyl phthalate can be used in the cosmetics, but the use of this substance in cosmetics, including nail polishes, is banned in the European Union under Directive 76/768/EEC 1976. [3] The use of Dibutyl phthalate has been restricted in the European Union for use in children's toys since 1999. An EU Risk Assessment has been conducted on Dibutyl phthalate and the final outcome has now been published in the EU Official Journal. To eliminate a potential risk to plants in the vicinity of processing sites and workers through inhalation, measures are to be taken within the framework of the IPPC Directive (96/61/EC) and the Occupational Exposure Directive (98/24/EC) Also includes the 2004 addendum. Antimicrobial efficacy of dibutyl phthalate has been reported from Streptomyces [4]. Dibutyl phthalate is also used as peroxisome proliferator, which is an effective compound against demodicidosis, as well as an endocrine disruptor with estrogenic activity, and a drug channeling agent.
Toxicity
The National Toxicology Program Center for the Evaluation of Risk to Human Reproduction (NTP-CERHR) has provided a summary of the toxicological data on dibutyl phthalate. US EPA (2006) has also summarized these data and included additional data published through February 2006. In the developing male fetus, the toxicological effects observed include a variety of malformations of the male reproductive system in structures that are dependent on properly functioning Leydig cells and the presence of adequate levels of testosterone. The malformations commonly observed in rats include hypospadias; decrease in anogenital distance; delayed preputial separation; agenesis of the prostate, epididymis, and vas deferens; degeneration of the seminiferous epithelium; interstitial cell hyperplasia of the testis; and retention of thoracic areolas or nipples. The underlying mode of action for these effects is inhibition of cholesterol transport and testosterone synthesis in the fetal Leydig cells.
Exposure to dibutyl phthalate at 50 mg/kg-day and above resulted in significant reductions in mRNA and protein concentration for proteins and enzymes involved in cholesterol transport and synthesis of testosterone, including scavenger receptor class B1 (SR-B1), steroidogenic acute regulated protein (StAR), P450 side chain cleavage enzyme (P450scc) and 3b-Hydroxysteroid dehydrogenase (3b-HSD). Mylchreest et al. gave pregnant Sprague-Dawley CD rats dibutyl phthalate by gavage in corn oil at 0, 0.5, 5, 50, or 100 mg/ kg-day (n = 19–20 per group) or 500 mg/kg-day (n = 11) on GDs 12–21. The day sperm was detected in the vaginal smear is defined as GD 0. In male offspring AGD was decreased at 500 mg/kg-day. A statistically significant increase (p < 0.05, using a nested analysis) in retained areolas or nipples on PND 14 was present in 31% and 90% of male pups at 100 and 500 mg/kg-day, respectively (80% and 100% of litters affected, respectively). [5]
References
[1]Huang, Jingyu; Nkrumah, Philip N.; Li, Yi; Appiah-Sefah, Gloria (2013). Reviews of Environmental Contamination and Toxicology Volume 224. Reviews of Environmental Contamination and Toxicology. 224. Springer, New York, NY. pp. 39–52.
[2]Kjeldsen, Peter; Barlaz, Morton A.; Rooker, Alix P.; Baun, Anders; Ledin, Anna; Christensen, Thomas H. (1 October 2002). "Present and Long-Term Composition of MSW Landfill Leachate: A Review". Critical Reviews in Environmental Science and Technology. 32 (4): 297–336.
[3]EU Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products
[4]Lee, D.S., 2000. Dibutyl phthalate, a glucosidase in- hibitor from Streptomyces melanosporofaciens. J. Biosci. Bioeng. 89, 271–273.
[5]Mylchreest, E., Wallace, D., Cattley, R., Foster, P., 2000. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n- butyl) phthalate during late gestation. Toxicol. Sci. 55, 143–151.
You may like
Related articles And Qustion
See also
Lastest Price from Dibutyl phthalate manufacturers

US $0.00/kg2025-06-23
- CAS:
- 84-74-2
- Min. Order:
- 1000kg
- Purity:
- i
- Supply Ability:
- 60tons

US $0.00/Kg/Drum2025-04-21
- CAS:
- 84-74-2
- Min. Order:
- 200KG
- Purity:
- 99%min
- Supply Ability:
- 500tons/month