Acyclovir: Applications and side effects
General description
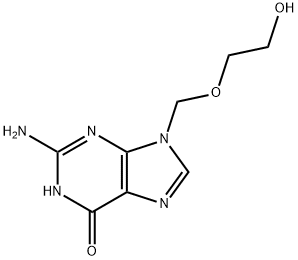
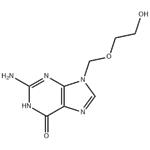
Aciclovir (ACV), also known as acyclovir, is an antiviral medication.[1] It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein-Barr virus infection. It can be taken by mouth, applied as a cream, or injected. Common side effects include nausea and diarrhea. Potentially serious side effects include kidney problems and low platelets. Greater care is recommended in those with poor liver or kidney function. It is generally considered safe for use in pregnancy with no harm having been observed. It appears to be safe during breastfeeding. Aciclovir is a nucleoside analogue that mimics guanosine. It works by decreasing the production of the virus's DNA. Its appearance is as follows:

Figure 1 Appearance of Acyclovir
Applications
Aciclovir is used for the treatment of herpes simplex virus (HSV) and varicella zoster virus infections, including: Its effectiveness in treating Epstein-Barr virus (EBV) infections is less clear. It has not been found to be useful for infectious mononucleosis due to EBV.[2] Valaciclovir and acyclovir act by inhibiting viral DNA replication, but as of 2016 there was little evidence that they are effective against Epstein–Barr virus, they are expensive, they risk causing resistance to antiviral agents, and (in 1% to 10% of cases) can cause unpleasant side effects. Aciclovir taken by mouth does not appear to decrease the risk of pain after shingles.[3] In those with herpes of the eye, aciclovir may be more effective and safer than idoxuridine. It is not clear if aciclovir eye drops are more effective than brivudine eye drops.
Intravenous aciclovir is effective to treat severe medical conditions caused by different species of the herpes virus family, including severe localized infections of herpes virus, severe genital herpes, chickenpox and herpesviral encephalitis. It is also effective in systemic or traumatic herpes infections, eczema herpeticum and herpesviral meningitis. Reviews of research dating from the 1980s show there is some effect in reducing the number and duration of lesions if aciclovir is applied at an early stage of an outbreak. Research shows effectiveness of topical aciclovir in both the early and late stages of the outbreak as well as improving methodologically and in terms of statistical certainty from previous studies.[4] Aciclovir trials show that this agent has no role in preventing HIV transmission, but it can help slow HIV disease progression in people not taking anti-retroviral therapy (ART). This finding emphasizes the importance of testing simple, inexpensive non-ART strategies, such as aciclovir and cotrimoxazole, in people with HIV.
Side effects
Common adverse drug reactions (≥1% of patients) associated with systemic aciclovir therapy (oral or IV) include nausea, vomiting, diarrhea, encephalopathy (with IV use only), injection site reactions (with IV use only) and headache. In high doses, hallucinations have been reported. Infrequent adverse effects (0.1–1% of patients) include agitation, vertigo, confusion, dizziness, oedema, arthralgia, sore throat, constipation, abdominal pain, hair loss, rash and weakness. Rare adverse effects (<0.1% of patients) include coma, seizures, neutropenia, leukopenia, crystalluria, anorexia, fatigue, hepatitis, Stevens–Johnson syndrome, toxic epidermal necrolysis, thrombotic thrombocytopenic purpura and anaphylaxis. Intravenous aciclovir may cause reversible nephrotoxicity in up to 5% to 10% of patients because of precipitation of aciclovir crystals in the kidney. Aciclovir crystalline nephropathy is more common when aciclovir is given as a rapid infusion and in patients with dehydration and preexisting renal impairment. Adequate hydration, a slower rate of infusion, and dosing based on renal function may reduce this risk.[5] The aciclovir metabolite 9-carboxymethoxymethylguanine (9-CMMG) has been shown to play a role in neurological adverse events, particularly in older people and those with reduced renal function.
References
[1]de Clercq, Erik; Field, Hugh J (5 October 2005). "Antiviral prodrugs – the development of successful prodrug strategies for antiviral chemotherapy". British Journal of Pharmacology. 147 (1). Wiley-Blackwell (published January 2006). pp. 1–11. doi:10.1038/sj.bjp.0706446. PMC 1615839. PMID 16284630.
[2]Gershburg, E; Pagano, JS (August 2005). "Epstein-Barr virus infections: prospects for treatment". The Journal of Antimicrobial Chemotherapy. 56 (2): 277–81. CiteSeerX 10.1.1.320.6721. doi:10.1093/jac/dki240. PMID 16006448.
[3]Chen, N; Li, Q; Yang, J; Zhou, M; Zhou, D; He, L (Feb 6, 2014). "Antiviral treatment for preventing postherpetic neuralgia". The Cochrane Database of Systematic Reviews. 2 (2): CD006866. doi:10.1002/14651858.CD006866.pub3. PMID 24500927.
[4]Spruance SL, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T (2002). "Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials". Antimicrob. Agents Chemother. 46 (7): 2238–43. doi:10.1128/aac.46.7.2238-2243.2002. PMC 127288. PMID 12069980.
[5]Razonable, RR (October 2011). "Antiviral drugs for viruses other than human immunodeficiency virus". Mayo Clinic Proceedings. 86 (10): 1009–26. doi:10.4065/mcp.2011.0309. PMC 3184032. PMID 21964179.
You may like
Related articles And Qustion
See also
Lastest Price from Acyclovir manufacturers
US $0.00/kg2025-11-25
- CAS:
- 59277-89-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/kg2025-09-29
- CAS:
- 59277-89-3
- Min. Order:
- 1kg
- Purity:
- 98.5%-101.0%;USP
- Supply Ability:
- 1000KGS