Dexamethasone: The Multifaceted Marvel in Medicine
Introduction
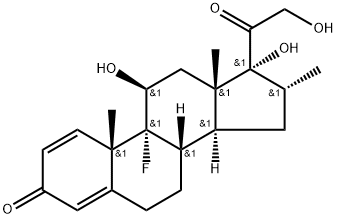
Dexamethasone is a glucocorticosteroid used in the treatment of different inflammatory conditions related to allergic disorders, skin conditions and lupus, psoriasis, ulcerative colitis, arthritis and respiratory disorders. Dexamethasone is also used either alone or in combination with other drugs to treat some types of cancers or to prevent/treat cancer-related conditions.

Mode of action
Dexamethasone (a synthetic glucocorticoid) has previously been used to treat asthma, allergic reactions, arthritis, and other autoimmune diseases. It acts through the blockade of two pathways of inflammation: vasodilation and immune cell migration. Dexamethasone crosses the host cell membrane and binds to glucocorticoid receptors present in the cell cytoplasm, which initiates a series of immune cell responses that lead to pro-inflammatory suppression cytokines IL-1, IL-2, IL-6, IL-8, TNF, and IFN-γ through a decrease in gene transcription. Out of these pro-inflammatory cytokines, five are associated with COVID-19 progression. It also increases the gene expression of IL-10, which is an anti-inflammatory cytokine mediator and inhibits neutrophil adhesion to endothelial cells, thus preventing the release of lysosomal enzymes and preventing chemotaxis at the site of inflammation. They also inhibit macrophages activation, one of the significant perpetrators of cytokine storms in COVID-19-infected individuals.
A breakthrough in Covid-19 therapy
A ground-breaking development in the fight against COVID-19 came from the Randomized Evaluation of COVID-19 therapy Trial on June 16, 2020. The Oxford RECOVERY Trial tested low-dose dexamethasone, lopinavir-ritonavir, hydroxychloroquine, and azithromycin in a randomized manner. Only dexamethasone succeeded in reducing the COVID-19-associated mortality rate. The use of dexamethasone at a dosing rate of 6 mg per day was started on June 8, 2020, for ten days to evaluate the clinical effectiveness in patients compared to 4321 patients with usual care alone. Dexamethasone reduced the death rate by one-third in patients on a ventilator and by one-fifth in patients on oxygen support. Early findings showed that dexamethasone decreased the mortality risk from 40% to 28% in ventilated patients and 25%–20% for patients on oxygen therapy over 28 days. Dexamethasone use did not cause any significant side effects and was ineffective in mild cases. However, The Oxford RECOVERY Trial has limitations; the results for critical secondary outcomes, potential adverse effects, and efficacy of dexamethasone in patients with comorbidities have not been reported. The current administration of corticosteroids should be limited to patients with severe conditions related to cytokine storm, including ARDS, renal failure, acute cardiac injury, and elevated serum levels of D-dimers.
A study conducted in Wuhan, China, demonstrated that corticosteroid use in patients with ARD reduced death risk, and 23 out of 50 (46%) patients who received corticosteroids died compared to 61.8% who did not receive corticosteroids. A laboratory study on dexamethasone in coronavirus infected pigs revealed the effectiveness of treatment in the acute phase of the disease, and rapid viral replication was observed with prolonged use of dexamethasone. On the contrary, there have been reports of corticosteroids associated with immunity suppression increased viral load, and delayed clearance from the body. Chu et al. (2004) indicated increased number of viruses after corticosteroid treatment in SARS-CoV-2 patients. Despite the numerous published data on the use of steroids in COVID-19, there is no clear evidence of corticosteroid treatment effectiveness in SARS. Some researchers emphasize the need for cautious use of corticosteroids, but randomized controlled trials are required to approve the positive effects and predict the optimal dosage regimen. However, the possible beneficial effects should be calculated compared to the risks, including secondary infections and delayed viral clearance. On September 2, 2020, WHO issued a guideline on the use of dexamethasone and other corticosteroids (hydrocortisone or prednisone) for the treatment of Covid-19.in the guideline, corticosteroids use was recommended in severe and critical patients.
References:
[1] AITEBIREMEN GIFT OMOKHUA-UYI J V S. Natural product remedies for COVID-19: A focus on safety[C]//Vol. 139. 2021: 1-494. DOI:10.1016/j.sajb.2021.03.012.[2] SOBIA NOREEN A M Irsah Maqbool. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic[J]. European journal of pharmacology, 2021, 894. DOI:10.1016/j.ejphar.2021.173854.
You may like
Related articles And Qustion
Lastest Price from Dexamethasone manufacturers

US $0.00/kg2025-08-15
- CAS:
- 50-02-2
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 10000

US $5.00-0.50/KG2025-05-30
- CAS:
- 50-02-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS