Description, Synthesis and Function of 4-(aminomethyl) benzoic acid
General description
The density of 4-(aminomethyl) benzoic acid is 1.2±0.1 g/cm3, the melting point is over 300 °C(lit.), the boiling point is 323.1±25.0 °C at 760 mmHg [1], the flash point is 149.2±23.2 °C, the exact mass is 151.063324, the PSA is 63.32000, the LogP is 0.76, the vapour pressure is 0.0±0.7 mmHg at 25 °C [2], the index of refraction is 1.598, and the storage condition is at 0-5 °C [3].
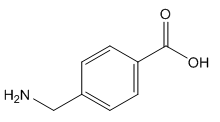
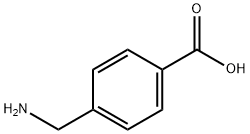
Fig. 1 The structure of 4-(aminomethyl) benzoic acid.
Synthesis
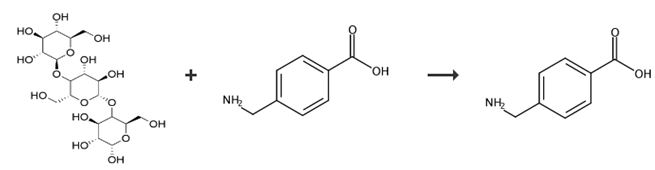
Fig. 2 The synthesis of 4-(aminomethyl) benzoic acid [4].
To a 15 mL tube containing
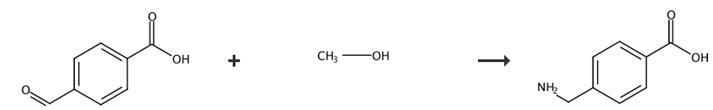
Fig. 3 The synthesis of 4-(aminomethyl) benzoic acid [5].
(1) Into a reaction vessel, 4-formyl benzoic acid (1) 1.00g (Ald, Lot. 12928MC) and methanol 5.00mL (5v/w) were added, heated to an external temperature of 80~90 °C on an oil bath and refluxed for 3 hours. The reaction solution was cooled approximately to 30 °C. An aliquot of the reaction solution was sampled and then concentrated to dryness. The concentrated residue was dissolved in D2O and completion of the reaction was confirmed from the generation of 4-dialkoxy methyl benzoic acid (2) by NMR. NMR (D2O) δ: 7.59 (2H, d), 7.19 (2H, d), 5.15 (1H, s), 3.10 (6H, s).
(2) To the above obtained reaction mixture, sodium hydroxide 0.27g (1eq), 28% aqueous ammonia 4.5mL, 5% Ru-C50mg were plced in an autoclave and hydrogen 10 kg/cm2 (opening pressure) was blown into the mixture at an external temperature of 150~160 °C (pressure upon heating 21kg/cm2) and the reaction was carried out for 9 hours. The catalyst was filtered off, the reaction mixture was concentrated to dryness, the residue was analysed by HPLC (column carrier: octadecylsilane) and the generated 4-Amino methyl benzoic acid (3) 0.95g (yield 93.6%) was confirmed. In addition, as byproducts, 4-hydroxymethyl benzoic acid 3.6% and 4-methyl benzoic acid 2.5% were generated. Furthermore, some of the above residue was dissolved in D2O and was measured by NMR. NMR (D2O) δ: 7.64 (2H, d), 7.20 (2H, d), 3.62 (2H, s).
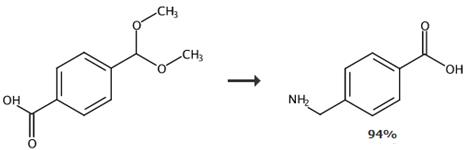
Fig. 4 The synthesis of 4-(aminomethyl) benzoic acid [6].
To the above obtained reaction mixture, sodium hydroxide 0.27g (1eq), 28% aqueous ammonia 4.5mL, 5% Ru-C50 mg were plced in an autoclave and hydrogen 10 kg/cm2 (opening pressure) was blown into the mixture at an external temperature of 150~160 °C (pressure upon heating 21kg/cm2) and the reaction was carried out for 9 hours. The catalyst was filtered off, the reaction mixture was concentrated to dryness, the residue was analysed by HPLC (column carrier: octadecylsilane) and the generated 4-Amino methyl benzoic acid (3) 0.95g (yield 93.6%) was confirmed. In addition, as byproducts, 4-hydroxymethyl benzoic acid 3.6% and 4-methyl benzoic acid 2.5% were generated. Furthermore, some of the above residue was dissolved in D2O and was measured by NMR. NMR (D2O) δ: 7.64 (2H, d), 7.20 (2H, d), 3.62 (2H, s).
Application
4-(Aminomethyl)benzoic acid is also called 4-carboxybenzylamine, which can protect blood platelet in extracorporal circulation, decrease hemorrhage, and guard against the secondary hyphema [7]. As a hemostatic compared with amino-methyl caproic acid, it has the merits of swift hemostasia, long action time and the low frequency of the dyspepsia. 4-(Aminomethyl)benzoic acid has many other clinical medicinal effects. Used with other medicament, it can defend teeth, take pre-cautions against ultraviolet radiation, and avoid derma-tosis.
Calorimetric study and thermal analysis
Molar heat capacities of 4-(aminomethyl)benzoic acid were precisely measured with a small sample precision automated adiabatic calorimeter over the temperature range from 80 to 400 K, and the heat capacity data were fitted to a polynomial equation: Cp,m=162.01+91.771X+6.4372X2+1.7517X3−4.991X4+8.909X5+1.585X6, X= (T−235)/155. The constant-volume energy of combustion of the compound was measured by means of a precision oxygen bomb combustion calorimeter [8]. The standard enthalpy values of combustion and formation were determined to be: ΔcHm= −(4300±4) kJ·mol−1 and ΔfHm= −(420±5) kJ·mol−1 [9].
Transportation precautions
The railway transportation shall be carried out in strict accordance with the dangerous goods packing list. Before transportation, check whether the packaging container is complete and sealed, and ensure that the container does not leak, collapse, fall, and damage during transportation. It is strictly forbidden to mix with acids, oxidants, food and food additives. Transport vehicles should be equipped with leakage emergency treatment equipment. During transportation, it should be protected from sun exposure, rain and high temperature.
Storage precautions
Store in a cool, ventilated warehouse. Keep away from fire and heat source. Packing sealed. It should be stored separately from oxidants, acids and edible chemicals, and must not be mixed. The storage area shall be equipped with suitable materials to accommodate leaks. The "five pairs" management system for extremely toxic substances should be strictly implemented.
References
[1] P. Groth, O. Hassel, Cis-trans Relationship Between the Two Amino Acids Obtained by Hydrogenation of p-Aminomethyl Benzoic Acid, Acta chem. scand 19(7) (1965).
[2] M.M. Khodaei, A. Alizadeh, M. Haghipour, Preparation and characterization of isatin complexed with Cu supported on 4-(aminomethyl) benzoic acid-functionalized Fe3O4 nanoparticles as a novel magnetic catalyst for the Ullmann coupling reaction, Research on Chemical Intermediates 45(5) (2019) 2727-2747.
[3] M.J. Kullman, S. Molesworth, G. Berden, J. Oomens, M. Van Stipdonk, IRMPD spectroscopy b2 ions from protonated tripeptides with 4-aminomethyl benzoic acid residues, International Journal of Mass Spectrometry 316 (2012) 174-181.
[4] H. LANDMANN, F. MARKWARDT, H.-G. KAZMIROWSKI, P. NEULAND, Zusammenhänge zwischen chemischer Konstitution und Antitrypsinwirkung bei Derivaten der 4-Aminomethyl-benzoesäure und anderen strukturverwandten cyclischen Verbindungen, (1967).
[5] R. Pascal, R. Sola, F. Labéguère, P. Jouin, Synthesis of the novel amino acid 4‐amino‐3‐(aminomethyl) benzoic acid (AmAbz) and its protected derivatives as building blocks for pseudopeptide synthesis, European Journal of Organic Chemistry 2000(22) (2000) 3755-3761.
[6] Z. Shang, L. Jia, M. Li, W. Hu, Y. Zhao, X. Wang, S. Wu, Solution thermodynamic analysis of p-(aminomethyl) benzoic acid in four binary solvents from 288.15 to 328.15 K, Journal of Chemical & Engineering Data 63(12) (2018) 4720-4734.
[7] M. Shipchandler, J. Fino, L. Klein, C. Kirkemo, 4′-[Aminomethyl] fluorescein and its N-alkyl derivatives: useful reagents in immunodiagnostic techniques, Analytical biochemistry 162(1) (1987) 89-101.
[8] W. WENNER, 4-Aminomethyl-4'-aminodiphenylsulfone and Related Compounds, The Journal of Organic Chemistry 22(11) (1957) 1508-1513.
[9] Q. Zheng, Z. Tan, D. Wang, A. Hao, B. Liu, X. LÜ, Q. Shi, Calorimetric Study and Thermal Analysis of 4‐(Aminomethyl) Benzoic Acid, Chinese Journal of Chemistry 27(4) (2009) 672-676.
See also
Lastest Price from 4-(Aminomethyl)benzoic acid manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 56-91-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 800kg

US $0.00/Kg/Drum2025-04-21
- CAS:
- 56-91-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt