Description, Synthesis, Function of Avanafil
General description
Avanafil, a phosphodiesterase 5 (PDE5) inhibitor, is a research project transferred by Vivus from Mitsubishi Tanabe pharmaceutical company in Japan [1]. It is used to treat male erectile dysfunction (ED). Avanafil is a medication that was recently approved by the US Food and Drug Administration for the management of erectile dysfunction. Avanafil is a new phosphodiesterase type 5 inhibitor similar to sildenafil and tadalafil. Avanafil was studied in over 1300 patients during clinical trials, including patients with diabetes mellitus and those who had undergone radical prostatectomy, and was found to be more effective than placebo in all men who were randomized to the drug [2]. The medication was studied with on-demand dosing that may occur after food and/or alcohol. Avanafil is dosed as 50 mg, 100 mg, or 200 mg tablets. Avanafil may differentiate itself from the other phosphodiesterase type 5 inhibitors with its quicker onset and higher specificity for phosphodiesterase type 5 versus other phosphodiesterase subtypes, but may lead to complications of therapy [3].
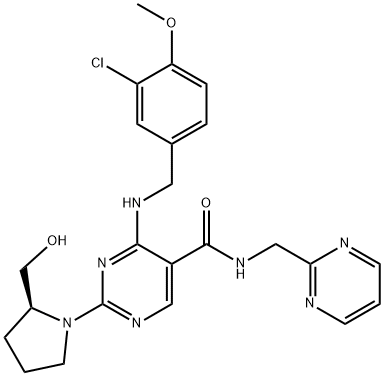
Avanafil(4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide;(S)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[(2-pyrimidinylmethyl) carbamoyl]pyrimidine) has a molecular weight of 483.95 Da and is a pyrimidine derivative that exists as a single enantiomer with S stereochemistry. It appears as a white crystalline powder in its pure form and is minimally soluble in water and moderately soluble in organic solvent. Avanafil is more soluble in acidic buffers (about pH 4) and is much less soluble in neutral and alkaline buffers [4]. Avanafil works in penile tissue to increase the level of cyclic guanosine monophosphate (cGMP). As levels of cGMP increase, smooth muscles relax in the penis, increasing local blood flow, which results in an erection. However, erection is only attained if stimulation occurs [5].
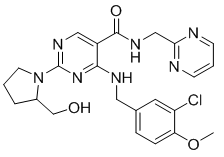
Fig. 1 Structural formula of avanafil.
Synthesis
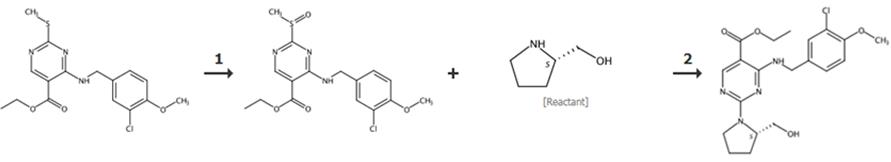
Fig. 2 The synthetic route of vardenafil [6].
(1) Synthesis of M1: Add SM2 and an appropriate amount of H2O into the three necked bottle, stir to dissolve, add excess Na2CO3, heat the water bath to 20-30 ° C, add 2 equivalent SM1 after dissolving, the system is light yellow green, there is a large amount of solid precipitation, and stir overnight. Adjust the pH with concentrated hydrochloric acid, produce a large amount of gas, filter and rinse, and dry the filter cake with air blast to obtain white solid, with a yield of 84%.
(2) Synthesis of M2: Add M1 and toluene into a three necked bottle, raise the temperature to 90-100 ° C, drop excess POCl3, raise the temperature to 90 ° C to obtain a yellow turbid solution, react for 2-3H, and gradually reduce to room temperature. Drop water into the reaction solution, speed up the stirring, stand for layering, separate the organic phase, wash it with alkaline aqueous solution, and then separate the toluene solution of the organic phase m2 for use.
(3) Synthesis of M3: Add SM3 and triethylamine to the toluene solution of M2 for reaction, add water and stir, stand for separation, separate the organic phase, wash and separate the organic phase, and concentrate under reduced pressure to obtain the mixture. The above mixture was dissolved in solvent and crystallized to obtain a white solid with a yield of 64%.
(4) Synthesis of M4: Add M3 to dichloromethane, cool down, slowly add m-chloroperoxybenzoic acid (mcpba), and then react for 1-2h. After the reaction is completed, quench and separate the methylene chloride solution of M4 for use.
(5) Synthesis of M5: Add SM4 and triethylamine to the dichloromethane solution of M4 and react for 1 - 3H. Add Na2CO3 solution into the reaction liquid, stir it fully, stand still to separate the liquid, separate the organic phase, evaporate it under reduced pressure, and get the oil for use.
(6) Synthesis of M6: Add NaOH solution to the above oil containing M5, heat it up and heat it overnight. After the reaction was completely filtered, the pH was adjusted with dilute hydrochloric acid to obtain a white solid. After purification and drying, M6 pure product was obtained with a yield of 72%.
(7) Synthesis of avanafil: Add M6, EDCI, HOBt, DCM, and sm5 into a three mouth bottle and react at room temperature for 10-15 H. After quenching reaction, the organic phase was separated and evaporated to dryness, solvent crystallization was added, and a large number of white solids were obtained after filtration and drying, with a yield of 57%.
Clinical data
Avanafil, administered in doses of 50 to 200 mg (maximum dose), is rapidly absorbed, the median time to maximum plasma concentration (Tmax) is 30–45 min in the fasting state and 1 h 20 min when the drug is taken together with a high fat meal. Owing to clearance by the hepatic metabolism, avanafil is degraded into two major metabolites, designated as M4 and M16. The M4 metabolite accounts for approximately 23% of the parent compound and 4% of its pharmacologic activity, with an in vitro inhibitory potency for PDE5 of 18% of that of avanafil. The M16 metabolite, accounting for approximately 29% of the parent compound, is inactive against PDE5. In phase I settings to investigate the tolerability and pharmacokinetic properties of the drug, avanafil was shown to be rapidly absorbed after oral administration, with a Tmax of approximately 35–45 min [7]. With regard to the elimination time from the plasma, discordant data have been presented: the apparent T1/2 was estimated within a range from 1 h 10 min to 1 h 20 min to a maximum of 5 h, as well as even 11 h (sildenafil: 3–4 h; vardenafil: 4–5 h; tadalafil: 17.5 h). Although these values were similar among the different dose units administered to the volunteers in the respective studies (50 mg, 100 mg and 200 mg), it remains to be elucidated whether or not the T1/2 can be considered a pharmacokinetic property that distinguishes avanafil from [8].
Usage
Different dosage forms and specifications of this product may vary, please read the specific drug instructions or follow the doctor's advice.
Adult oral 100mg once a day.
According to the tolerance, the dosage of this product can be adjusted to 50mg or 200mg. The Lowest effective dose should be used.
It should be combined with moderate CYP3A4 inhibitors and α -receptor antagonists, no more than 50mg per day, and should not be combined with strong CYP3A inhibitors [9].
Adverse reactions
Common headache, nasopharyngitis, flushed face, nasal congestion, lumbago and muscle pain.
Rare upper respiratory tract infection, bronchitis, influenza, sinusitis, hypertension, dyspnea, nausea, constipation, rash [10].
Other PDE3 may cause non-arteritic anterior ischemic optic neuropathy (NAION) and insomnia. Patients with heart disease, diabetes, hypertension, hyperlipidemia and certain eye diseases, as well as those who smoke or are older than 50, have a higher chance of developing NAION [11].
Taboo
Patients allergic to this product are prohibited.
Do not use in patients who are taking nitrates.
Women are forbidden.
The safety and efficacy of patients under 18 years of age have not been determined.
Matters needing attention
Concomitant use of this product with nitrates may cause hypotension, dizziness, syncope, heart attack or stroke.
Ssexual activity can increase the risk of cardiovascular disease, cardiovascular disease should pay attention to [12].
If erection exceeds 4h, please seek medical attention with or without pain.
Seek medical advice immediately if vision or hearing loss occurs during the use of this product.
Drug interaction
This product can obviously enhance the antihypertensive effect of nitric ester drugs.
Combined with a receptor antagonist, the antihypertensive effect is increased, can cause hypotension.
Drinking alcohol during the use of this product may increase the risk of postural hypotension [13].
Concomitant use of this product with strong CYP3A4 inhibitors such as ketoconazole and ritonavir can significantly increase blood drug concentration, so their concomitant use is prohibited.
CYP3A4 inhibitor can increase the blood concentration of this product by 2-3 times, and the dosage should be reduced when combined [14].
Concomitant use of this product with CYP3A4 inducer is forbidden.
There was no clinically significant effect on the pharmacokinetics of amlodipine, omeprazole, warfarin, dixipamine and rosiglitazone [15].
Storage method
Storage at 20-25℃, short-range carrying allowed 15-30℃.
Present situation
Avanafil is a new medication in the phosphodiesterase type 5 inhibitor class. The manufacturers plan to market avanafil as the first agent in the second generation of this class with a more rapid onset and a better side effect profile. It is apparent that avanafil is effective for the treatment of erectile dysfunction and from the 1300 patients who have taken the medication during clinical trials, it appears to be as safe as the other available agents in the class [16]. However, it is unclear what role this agent will have in the four billion dollar per year marketplace for medications to treat erectile dysfunction. Previously, all of the agents in this class were similarly priced, but with sildenafil potentially becoming generically available later this year in some markets, patients may decide that the slight benefits of avanafil are not worth its extra cost [17].
References
[1] A. Alwaal, R. Al-Mannie, S. Carrier, Future prospects in the treatment of erectile dysfunction: focus on avanafil, Drug design, development and therapy 5 (2011) 435.
[2] L. Belkoff, A. McCullough, I. Goldstein, L. Jones, C. Bowden, K. DiDonato, B. Trask, W. Day, An open‐label, long‐term evaluation of the safety, efficacy and tolerability of avanafil in male patients with mild to severe erectile dysfunction, International journal of clinical practice 67(4) (2013) 333-341.
[3] L. Boeri, P. Capogrosso, E. Ventimiglia, A. Serino, G. La Croce, A. Russo, R. Damiano, F. Montorsi, A. Salonia, Avanafil-a further step to tailoring patient needs and expectations, Expert Review of Clinical Pharmacology 9(9) (2016) 1171-1181.
[4] N.Ö. Can, Development of validated and stability-indicating LC-DAD and LC-MS/MS methods for determination of avanafil in pharmaceutical preparations and identification of a novel degradation product by LCMS-IT-TOF, Molecules 23(7) (2018) 1771.
[5] G. Corona, G. Rastrelli, A. Burri, E.A. Jannini, M. Maggi, The safety and efficacy of Avanafil, a new 2nd generation PDE5i: comprehensive review and meta-analysis, Expert opinion on drug safety 15(2) (2016) 237-247.
[6] I. Goldstein, L.A. Jones, L.H. Belkoff, G.S. Karlin, C.H. Bowden, C.A. Peterson, B.A. Trask, W.W. Day, Avanafil for the treatment of erectile dysfunction: a multicenter, randomized, double-blind study in men with diabetes mellitus, Mayo Clinic Proceedings, Elsevier, 2012, pp. 843-852.
[7] J. Jung, S. Choi, S.H. Cho, J.-L. Ghim, A. Hwang, U. Kim, B.S. Kim, A. Koguchi, S. Miyoshi, H. Okabe, Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: a single-and multiple-dose, double-blind, randomized, placebo-controlled, dose-escalation study in healthy Korean male volunteers, Clinical therapeutics 32(6) (2010) 1178-1187.
[8] J. Kotera, H. Mochida, H. Inoue, T. Noto, K. Fujishige, T. Sasaki, T. Kobayashi, K. Kojima, S. Yee, Y. Yamada, Avanafil, a potent and highly selective phosphodiesterase-5 inhibitor for erectile dysfunction, The Journal of urology 188(2) (2012) 668-674.
[9] M. Kurakula, O.A. Ahmed, U.A. Fahmy, T.A. Ahmed, Solid lipid nanoparticles for transdermal delivery of avanafil: optimization, formulation, in-vitro and ex-vivo studies, Journal of liposome research 26(4) (2016) 288-296.
[10] J.A. Kyle, D.A. Brown, J.K. Hill, Avanafil for erectile dysfunction, Annals of Pharmacotherapy 47(10) (2013) 1312-1320.
[11] M. Limin, N. Johnsen, W.J. Hellstrom, Avanafil, a new rapid-onset phosphodiesterase 5 inhibitor for the treatment of erectile dysfunction, Expert opinion on investigational drugs 19(11) (2010) 1427-1437.
[12] M. Sanford, Avanafil: a review of its use in patients with erectile dysfunction, Drugs & aging 30(10) (2013) 853-862.
[13] K.A. Soliman, H.K. Ibrahim, M.M. Ghorab, Effects of different combinations of nanocrystallization technologies on avanafil nanoparticles: in vitro, in vivo and stability evaluation, International journal of pharmaceutics 517(1-2) (2017) 148-156.
[14] D. Swearingen, A. Nehra, S. Morelos, C.A. Peterson, Hemodynamic effect of avanafil and glyceryl trinitrate coadministration, Drugs in Context 2013 (2013).
[15] C. Zhao, S.W. Kim, D.Y. Yang, J.-J. Kim, N.C. Park, S.W. Lee, J.S. Paick, T.Y. Ahn, K.H. Moon, W.S. Chung, Efficacy and safety of avanafil for treating erectile dysfunction: results of a multicentre, randomized, double-blind, placebo-controlled trial, BJU international 110(11) (2012) 1801-1806.
[16] M. Zhao, X. Wu, Z. Yu, Y. Sun, Z. Liu, J. Yuan, H. Liu, Y. Jin, Identification, synthesis and characterization of avanafil process impurities and determination by UPLC, RSC advances 12(15) (2022) 9256-9262.
[17] J.L. Zurawin, C.A. Stewart, J.E. Anaissie, F.A. Yafi, W.J. Hellstrom, Avanafil for the treatment of erectile dysfunction, Expert Review of Clinical Pharmacology 9(9) (2016) 1163-1170.
You may like
Related articles And Qustion
Lastest Price from Avanafil manufacturers

US $5.00/g2026-01-24
- CAS:
- 330784-47-9
- Min. Order:
- 100g
- Purity:
- 99%
- Supply Ability:
- 5000tons

US $0.00/kg2025-08-29
- CAS:
- 330784-47-9
- Min. Order:
- 1kg
- Purity:
- 99.0%
- Supply Ability:
- 500kg