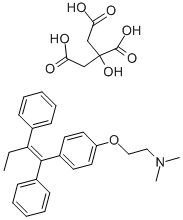
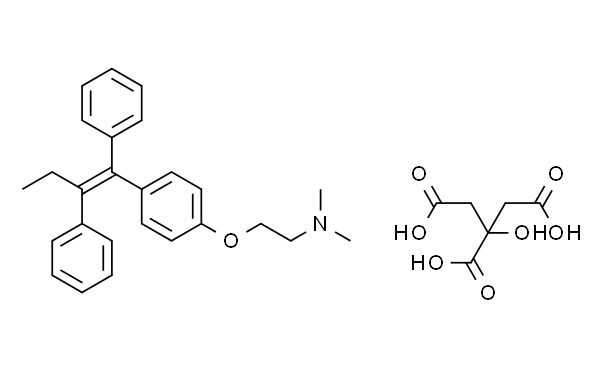
Description, clinical application and adverse reactions of tamoxifen citrate
General description
Tamoxifen citrate is an orally administered, nonsteroidal antiestrogen agent that is widely used for the treatment of breast cancer and that has recently been found to prevent breast cancer in some high-risk populations[1]. Additionally, tamoxifen, a non-steroidal antioestrogen, represents a significant advance in treatment of female breast cancer. Response rates to tamoxifen in advanced breast cancer are around 30 to 35%, increasing with patient selection for oestrogen receptor positivity[2]. Tamoxifen must be regarded as first-line endocrine treatment in postmenopausal women, and may represent an alternative to first-line ovarian ablation in premenopausal women. An emergent role in primary therapy of elderly and frail patients with operable disease is apparent. Tamoxifen is also of benefit following surgery in male breast cancer, and may have a role as first-line endocrine treatment[3]. Tamoxifen also has a potential role in other hormone-sensitive malignancies such as pancreatic carcinoma, and in treatment of benign breast disease. Finally, tamoxifen has a place in treatment of male and female infertility.
Pharmacodynamic Studies
Tamoxifen is the trans isomer of a triphenylethylene derivative, and is administered orally as the citrate salt. Tamoxifen exhibits complex pharmacological properties, acting as an oestrogen antagonist, or partial or full agonist, depending on the target tissue and the species studied[4]. Predominantly antioestrogenic and partial oestrogenic effects are apparent in humans and in rats, antioestrogenic effects in the chick, and fully oestrogenic effects in the mouse and in the dog. A number of metabolites are active; the N-demethyl metabolite may contribute to the activity of the parent compound in vivo[5].
The tumour growth inhibitory actions of tamoxifen have been investigated in vitro principally using the oestrogen-responsive MCF-7 human breast cancer cell line; in addition there has been extensive research using the DMBA-induced rat mammary carcinoma model. The precise mechanism of tamoxifen’s antitumour activity remains elusive[6-7]. The classical account involves competitive blockade by tamoxifen of sites on the oestrogen receptor; however, recently the emphasis of research has shifted to address effects of tamoxifen on tumour growth of which postulated mediators include polypeptide growth factors, a specific anti-oestrogen binding site, protein kinase C, and calmodulin[8].
Tamoxifen has complex effects at the hypothalamic-pituitary level, the clinical significance of which is unclear. An elevation in plasma oestrogen levels has been observed in premenopausal patients. Tamoxifen generally exerts weak oestrogen-like effects in postmenopausal patients, with reduction in circulating gonadotrophin and prolactin levels and increases in serum pregnancy zone protein and sex hormone-binding globulin[9-10]. Other effects reported, of uncertain clinical significance, include reduction in functional antithrombin III activity and increases in serum high density lipoprotein cholesterol; tamoxifen appears not to exert adverse effects on bone mineral content.
Pharmacokinetic Studies
Data regarding the pharmacokinetics of tamoxifen in humans are incomplete. Following administration of a single oral dose, maximum plasma concentrations of the parent drug and the demethyl metabolite are achieved within several hours[11]. However, with chronic administration, steady-state concentrations are not attained for 3 to 4 weeks. Tamoxifen is highly plasma protein bound at therapeutic concentrations. It undergoes extensive hepatic metabolism; N-demethylation is the principal metabolic pathway, with subsequent sidechain deamination to the primary alcohol. The fraction of the dose excreted as unchanged drug in urine is negligible. Biliary excretion is the main route of elimination; elimination appears to be biphasic, with an initial phase of 7 to 14 hours, and a terminal phase of around 7 days[12].
Therapeutic Trials
Response rates to tamoxifen in a small number of male patients with breast cancer were around 40%, with a benefit in survival apparent in a trial of tamoxifen following surgery. Survival benefit was also demonstrated in 2 small trials of tamoxifen in pancreatic carcinoma, while responses in small trials in prostatic, ovarian, renal and colorectal carcinomas have not been impressive, and preliminary data regarding the use of tamoxifen in malignant melanoma are inconclusive[13-14].
Ovulation is achieved in around 70% of patients with anovulatory infertility following tamoxifen treatment, with rates of subsequent pregnancy, where assessed, ranging from 15 to 60%. Administration of tamoxifen to men with idiopathic oligospermia has been associated with subsequent pregnancy rates in partners of 20 to 34%. There is at present considerable interest in the potential of tamoxifen in treatment of benign breast disease[15]. Resolution of symptoms was apparent in 70 to 90% of patients with mastalgia receiving tamoxifen, in 2 preliminary controlled trials, and further investigations are in progress.
Adverse reactions
Tamoxifen is very well tolerated, and discontinuation of therapy because of adverse effects is rarely necessary. The most frequent adverse effects are related to the drug’s anti-oestrogenic activity, and include hot flushes, nausea and/or vomiting, vaginal bleeding or discharge, and menstrual disturbances in premenopausal patients[16]. Thus, tamoxifen continues to play a major role in management of female breast cancer in both early and advanced stages of disease, with a place also in treatment of male breast cancer and of infertility.
Tamoxifen is in general very well tolerated, and discontinuation of therapy because of adverse effects is rarely necessary. The most frequent adverse effects include hot flushes, nausea and/or vomiting, and vaginal bleeding or discharge, while menstrual disturbances occur in a significant proportion of premenopausal patients[17-18]. Haematological changes such as thrombocytopenia and leucopenia are reported infrequently, but the relationship to treatment is uncertain. CNS disturbances including dizziness, lethargy and depression have also been described, although infrequently. Disease ‘flare’ is apparent in a number of patients early in therapy, but is not necessarily an indication for treatment withdrawal. Thromboembolic events have been reported very rarely, and in contrast, potentiation of the anticoagulant effect of warfarin; ocular disturbances, generally in association with very high dosages, have also been described in rare instances.
The adverse reactions of tamoxifen citrate include nausea, vomiting, diarrhea, menstrual disorders, vaginal bleeding, facial flushing, hair loss, rash, headache, dizziness, weight gain, edema, bone pain, and pain at the tumor. Long term and massive use of tamoxifen citrate can cause visual impairment and occasional leukopenia and thrombocytopenia[19-20]. Tamoxifen citrate has an effect on the fetus and should not be used by pregnant women. In addition, those with abnormal hemogram and liver and kidney function should be used with caution. 4. Occasionally, there is pruritus of perineum and increased leucorrhea. 5. Sometimes it can cause hypercalcemia, which occurs within 1 ~ 2 weeks.
Application
The antiestrogen tamoxifen citrate is the most common form of endocrine therapy in the United States. However, endocrine therapy also includes ovarian ablation in premenopausal women—either surgically, through irradiation to the ovaries, or by the use of a luteinizing hormone-releasing hormone agonist[21]. In postmenopausal women, inhibition of extraovarian estrogen synthesis by aromatase inhibitors is also an option, but its role as adjuvant therapy is not clear. Tamoxifen is effective in both premenopausal and postmenopausal women with hormone-sensitive (ER-positive) breast cancer. In the meta-analysis, 5 years of adjuvant therapy with tamoxifen reduced the 10-year proportional risk of recurrence by 47% and the proportional risk of mortality by 26%. In addition, the use of tamoxifen reduced the risk of contralateral breast cancer by 47% over the same period[22]. Tamoxifen has beneficial effects on bone density and on the total cholesterol concentration because of its partial estrogenic properties. But its use is also associated with postmenopausal symptoms—such as hot flashes and vaginal discharge—and an increased risk of uterine cancer and thromboembolic events.9 Nonetheless, tamoxifen's overall risk-benefit ratio is favorable, and it should be offered as adjuvant treatment to women with hormone-sensitive breast cancer.
Dosage and Administration
The recommended dosage of tamoxifen, in advanced breast cancer and as adjuvant therapy, is 10mg twice daily, administered orally, increasing to 20mg twice daily if no response. The optimal duration of adjuvant therapy remains to be established, but at present it appears that longer, or indeed indefinite, treatment may be desirable. Tamoxifen should not be administered during pregnancy[23-24].
References
[1] M.V. Alavikhorassani Moghadam, Methods and compositions for protease reporter assays and modulators, 712 North, Inc., USA . 2022, p. 97pp.
[2] E.L. Albert, S.N. Yaacob, G. Bahmanrokh, T. Miyazaki, Y. Shirosaki, C.A.C. Abdullah, Preparation and characterization of encapsulated tamoxifen citrate-magnetite nanoparticle via oil in water emulsion evaporation technique, Nanomed. Res. J. 6(4) (2021) 337-346.
[3] C. Bai, L. Wang, R. Yue, M. Chen, Clinical observation of triprillin combined with tamoxifen in treatment of uterine fibroids and its effect on serum levels of TGF-β1, VEGF and HIF-1α, Yaowu Pingjia Yanjiu 44(7) (2021) 108-112.
[4] N.S. Dey, Mechanistic Approach of Nano Carriers for Targeted in Cancer Chemotherapy: A Newer Strategy for Novel Drug Delivery System, Polymers (Basel, Switz.) 14(12) (2022) 2321.
[5] N. Gharibeh, L. Aghebati-Maleki, J. Madani, R. Pourakbari, M. Yousefi, J. Ahmadian Heris, Cell-based therapy in thin endometrium and Asherman syndrome, Stem Cell Res. Ther. 13(1) (2022) 33.
[6] D. Gozali, I. Sopyan, H. Hairunnisa, S.S. Marvita, Solubility enhancement and characterization of tamoxifen citrate using co-crystallization, Indones. J. Chem. 22(2) (2022) 478-490.
[7] T. Ichim, Immunotherapeutic methods and compositions for targeting cancer fibroblasts, Figene, LLC, USA . 2021, p. 73pp.
[8] T. Incecayir, S. Olgac, D.Y. Usta, Z.S. Teksin, Role of surfactants on dissolution behavior of tamoxifen, Dissolution Technol. 28(2) (2021) 1-10.
[9] L.M. Johnson, L.A. Li, S.A. Krovi, A. Van der Straten, Multi-drug formulations for subcutaneous biodegradable reservoir device, Research Triangle Institute, USA . 2021, p. 62pp.
[10] D. Kavya, V.K. Nadumane, Anti-cancer enzyme composition and method of preparation thereof, Jain Deemed-To-Be University , India . 2022, p. 30pp.
[11] K.I. Kohn, Smart dosing for cancer therapy, Optimdosing LLC, USA . 2022, pp. 21pp., Cont.-in-part of U.S. Ser. No. 127,352.
[12] G.C. Kuscu, C. Gurel, A. Buhur, F. Oltulu, L. Akman, T. Kose, N.U.K. Yavasoglu, A. Yavasoglu, The regulatory effects of clomiphene and tamoxifen on mTOR and LC3-II expressions in relation to autophagy in experimental polycystic ovary syndrome (PCOS), Mol. Biol. Rep. 49(3) (2022) 1721-1729.
[13] Z. Li, Efficacy and Safety of Liqi Sanjie Plaster Combined with Tamoxifen in the Treatment of Hyperplasia of Mammary Glands, Yaopin Pingjia 18(3) (2021) 54-56.
[14] A.D. Pinzon-Garcia, R. Sinisterra, M. Cortes, F. Mesa, S. Ramirez-Clavijo, Polycaprolactone nanofibers as an adjuvant strategy for Tamoxifen release and their cytotoxicity on breast cancer cells, PeerJ (2021) e12124.
[15] Z. Qi, J. Tian, C. Liu, H. Sun, Y. Yu, Clinical study on acupoint lontophoresis of modified xiaoyao loubei powder for mastoplasia of liver-depression and phlegm-congealing type, Xin Zhongyi 51(4) (2019) 248-250.
[16] M. Sakhi, A. Khan, I. Khan, Z. Iqbal, S.I. Khan, M.A. Khattak, F. Ullah, A. Aman, M. Alam, A new sensitive HPLC/UV method for simultaneous determination of paclitaxel, sorafenib and omeprazole in standard solutions and spiked plasma: Application to in-vitro and in-vivo evaluation of paclitaxel polymeric nanoformulations, Trop. J. Pharm. Res. 20(9) (2021) 1949-1959.
[17] M.N. Shahid, T.M. Khan, C.F. Neoh, Q.Y. Lean, A. Bukhsh, M. Karuppannan, Effectiveness of pharmacological intervention among men with infertility: A systematic review and network meta-analysis, Front. Pharmacol. 12 (2021) 638628.
[18] S. Sharma, B. Singh, D. Kalja, Formulation, characterization and drug release characteristics of Dextran-PLGA-based nanoparticles loaded with tamoxifen citrate, World J. Pharm. Res. 11(3) (2022) 1310-1324.
[19] S. Singhal, J. Mehta, N. Mehta, A. Mehta, Liquid biopsy yield enhancement, Northwestern University, USA . 2021, p. 22 pp.
[20] N. Sittisanguanphan, N. Paradee, A. Sirivat, Hyaluronic Acid and Graphene Oxide-incorporated Hyaluronic Acid Hydrogels for Electrically Stimulated Release of Anticancer Tamoxifen Citrate, J. Pharm. Sci. (Philadelphia, PA, U. S.) 111(6) (2022) 1633-1641.
[21] M. Ueda, S. Kinoshita, Y. Honma, K. Nishimaki, T. Kurose, K. Tsuji, TLR3 pathway inhibitor comprising bacitracin, anti-inflammatory composition and TLR3 pathway inhibitory method, Rohto Pharmaceutical Co., Ltd., Japan; Kyoto Prefectural University of Medicine . 2021, p. 23pp.
[22] M.A. Villetti, A.R. Clementino, I. Dotti, P.R. Ebani, E. Quarta, F. Buttini, F. Sonvico, A. Bianchera, R. Borsali, Design and Characterization of Maltoheptaose-b-Polystyrene Nanoparticles, as a Potential New Nanocarrier for Oral Delivery of Tamoxifen, Molecules 26(21) (2021) 6507.
[23] J. Zhang, C. Li, S. Zhang, X. Wu, L. Zhou, Industrial preparation of tamoxifen citrate, Beijing Jingfeng Pharmaceutical Shandong Co., Ltd., Peop. Rep. China . 2022, p. 14pp.
[24] L. Zhao, M. Wang, Y. Zhang, C. Wang, Y. Hong, Effect of Tiaogan Gushen Decoction on Th1 and Th2 Cell Immune Response in Patients with Luminal Breast Cancer Treated by Endocrine Therapy, Liaoning Zhongyiyao Daxue Xuebao 23(6) (2021) 184-188.
Related articles And Qustion
See also
Lastest Price from Tamoxifen citrate manufacturers

US $5.00/Box2025-07-30
- CAS:
- 54965-24-1
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 20000 boxes

US $10.00/box2025-07-25
- CAS:
- 54965-24-1
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 100000box