Dapoxetine is an effective drug for the treatment of premature ejaculation
Inroduction
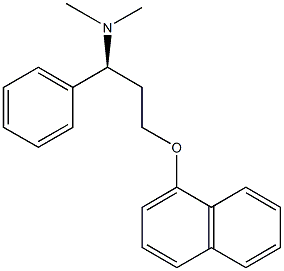
The sub formula of Dapoxetine is C21H23NO, white to off-white crystalline powder, molecular weight is 305.413, density is 1.081 g/cm³, CAS number is 119356-77-3, it can rapidly act and metabolize selective serotonin reabsorption Inhibitors (SSRIs), indicated for the treatment of premature ejaculation in men[1].
Dapoxetine, a selective serotonin reuptake inhibitor, is the first oral pharmacological agent indicated for the treatment of men aged 18–64 years with premature ejaculation. In four randomized, double-blind, placebo-controlled, multicentre studies of 12–24 weeks’ duration, oral dapoxetine 30 or 60 mg (administered as needed) was effective in the treatment of men with premature ejaculation, inducing significantly (p < 0.001) greater improvements from baseline than placebo in the primary efficacy endpoint (mean intravaginal ejaculatory latency time [IELT] or mean average IELT [defined as the average of IELT values over the previous 4 weeks], as measured by the female partner utilizing a stopwatch).
Picture 1 Dapoxetine tablets
The clinical studies and between dapoxetine and placebo
For the most part, dapoxetine recipients achieved significantly better outcomes than placebo recipients with regard to the secondary endpoints[2], including the Premature Ejaculation Profile (PEP) domains and the Clinical Global Impression or Patient Global Impression ratings of change in premature ejaculation, across these clinical studies.The beneficial effects of dapoxetine therapy on the perceived control over ejaculation and satisfaction with sexual intercourse PEP domains were sustained in a 9-month noncomparative extension phase of two identical 12-week, double-blind studies.Oral dapoxetine therapy for up to 12 months was generally well tolerated in men with premature ejaculation, with the nature of treatment-emergent adverse events generally similar across the clinical studies and between dapoxetine and placebo.
The safety of dapoxetine
Premature ejaculation (PE) is a major issue in male sexual health. The global prevalence of PE is estimated to be between 20% and 40%, making it the most common sexual dysfunction in men[3]. PE causes distress and reduced quality of life for patients and has a negative impact on interpersonal relationships. Historically, it has been treated with cognitive therapy, behavioral methods, and off-label use of selective serotonin reuptake inhibitors usually used to treat depression and other psychological disorders. Dapoxetine is a selective serotonin reuptake inhibitor specifically designed to treat PE. This paper reviews the current evidence for use of dapoxetine in the treatment of PE in adult men. There is substantial evidence that dapoxetine 30 mg or 60 mg taken “on-demand” results in a significant increase in intravaginal ejaculatory latency time when compared with placebo. Patient-reported outcomes are clearly improved relative to placebo following dapoxetine therapy, indicating greater control over ejaculation, more satisfaction with intercourse, less ejaculation-related distress, and, importantly, significantly reduced interpersonal difficulty. These data were supported by consistent reports of improvement in Clinical Global Impression of change in PE following treatment with dapoxetine. Further studies are needed to evaluate long-term efficacy and health economics. The unique pharmacology of dapoxetine makes it ideal for on-demand dosing, and the clinical evidence shows dapoxetine to be an efficacious and tolerable treatment for lifelong and acquired PE.
The pharmacokinetic studies of dapoxetine
Dapoxetine is being developed as a treatment for premature ejaculation and has demonstrated rapid absorption and elimination in previous pharmacokinetic studies. Two open-label studies were conducted in healthy men: a parallel-group pharmacokinetic and safety study in young and elderly men and a randomized crossover food-effect study. Maximal plasma dapoxetine concentrations (Cmax) were similar in young and elderly men (338 and 310 ng/mL, respectively), as were the corresponding area under the plasma concentration versus time curve (AUC) values (2040 and 2280 ng·h/mL, respectively). When coadministered with food, Cmax was reduced by 11% (398 vs 443 ng/mL in the fed and fasted states, respectively), and the peak was delayed by approximately 30 minutes, indicating that food slowed the rate of absorption; however, systemic exposure to dapoxetine (ie, AUC) was not affected by food consumption. Thus, age or consumption of a high-fat meal has only a modest impact on dapoxetine pharmacokinetics in healthy men.
Reference
1 McCarty E J, Dinsmore W W. Dapoxetine: an evidence-based review of its effectiveness in treatment of premature ejaculation[J]. Core evidence, 2012, 7: 1.
2 Dresser M J, Kang D, Staehr P, et al. Pharmacokinetics of dapoxetine, a new treatment for premature ejaculation: Impact of age and effects of a high‐fat meal[J]. The Journal of Clinical Pharmacology, 2006, 46(9): 1023-1029.
3 Jiann B P, Huang Y J. Assessing satisfaction in men with premature ejaculation after dapoxetine treatment in real‐world practice[J]. International Journal of Clinical Practice, 2015, 69(11): 1326-1333.
You may like
Related articles And Qustion
See also
Lastest Price from Dapoxetine manufacturers

US $0.00-0.00/KG2025-09-17
- CAS:
- 119356-77-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T

US $0.00/bag2025-05-21
- CAS:
- 119356-77-3
- Min. Order:
- 1bag
- Purity:
- 99
- Supply Ability:
- 1000