Crystal Structure of Titanium carbide
Titanium carbide (TiC) is a ceramic material with a high melting point (3160°C) and high hardness (9~9.5 in the Mosh scale). TiC is also an alternative non-precious electrocatalyst and carrier for fuel cells and electrolysers. Titanium carbide is a carbide with a wide homogeneity (from TiC 0.48 to TiC1.00). Synthesis conditions affect the ordered arrangement of vacancies in the carbon sublattice, leading to the appearance of non-stoichiometric TiCx, which results in the redistribution of carbon atoms and structural vacancies to form various ordered structures.
When the carbon atom vacancies are randomly distributed, the disordered TiC compounds form cubic NaCl crystal structures. When there is an ordered distribution of carbon atom vacancies, two stable and ordered phases of titanium carbide occur, a cubic phase and a triangular phase. TiC with a cubic NaCl crystal structure (Figure 1) is the most common titanium carbide phase.
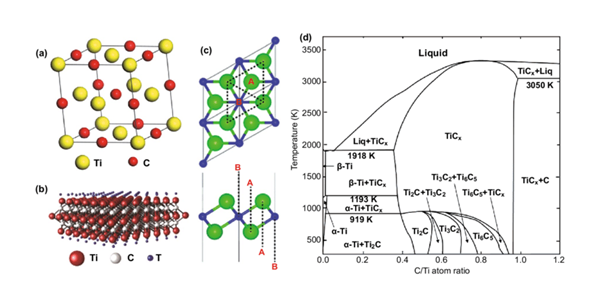
The ordered cubic phase Ti2C (space group Fd3m) has a lattice spacing twice that of the disordered titanium carbide.The Ti6C5 phase is a stable ordered phase and the unordered phase of all Group IV and Group V transition metal carbides. Ti8C5 is a rhombohedral crystal structure. There is also ordered titanium carbide of type 6H with fcc lattice.
Lastest Price from Titanium carbide manufacturers

US $0.00/kg2022-09-21
- CAS:
- 12070-08-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg