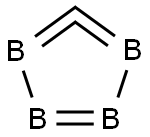
Crystal Structure of Boron carbide
Boron carbide (B₄C) is a high-temperature semiconductor that can be used in new electronic applications. B₄C is thermally stable, has a high melting point and hardness, a low density, is wear-resistant, and has remarkable ballistic properties. Therefore, it is widely used in industrial or military materials such as refractories, coatings and bulletproof vests.
The main structural units of Boron carbide are the 12 atomic icosahedra located at the vertices of the tripartite symmetric rhombic lattice (the R3m space group) and the 3-atom linear chain connecting the icosahedra along the (111) rhombic axis. As illustrated in Fig. 1.
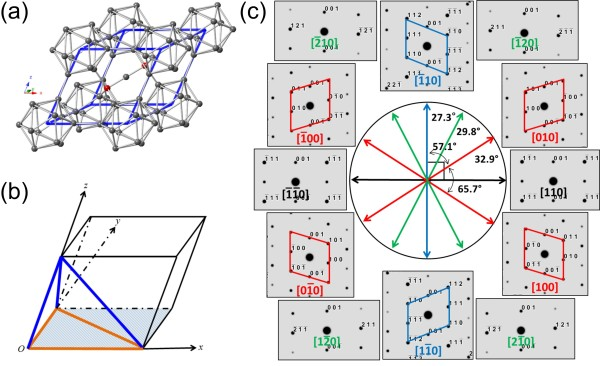
(a) In the rhombohedral lattice of boron carbide, eight 12-atom icosahedra are situated at the vertices, while a single 3-atom chain resides along the longest diagonal of the rhombohedron.
(b) The schematic drawing illustrates the rhombohedral unit cell, with the (001) plane highlighted in shading. Within this plane, the orange lines delineate the three in-zone directions: [100], [010], and [001], along which planar defects can be discerned. The blue lines indicate the three off-zone directions, which are not specified in the original text but would typically be perpendicular to the in-zone directions, and from these, planar defects are not observable.
(c) A roadmap is presented, composed of simulated diffraction patterns for the principal low index zone axes within the (001) plane. This roadmap serves as a guide during transmission electron microscopy (TEM) examination, assisting the operator in ascertaining the feasibility of tilting the specimen to achieve the desired zone axes.
Related articles And Qustion
Lastest Price from Boron carbide manufacturers
US $0.10/KG2024-08-21
- CAS:
- 12069-32-8
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000Tons

US $890.00/T2024-04-28
- CAS:
- Min. Order:
- 0.1T
- Purity:
- 98%
- Supply Ability:
- 20T