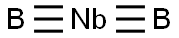Crystal structure and uses of Niobium boride
Niobium boride is corrosion-resistant to molten Ta while corroded by molten rhenium.
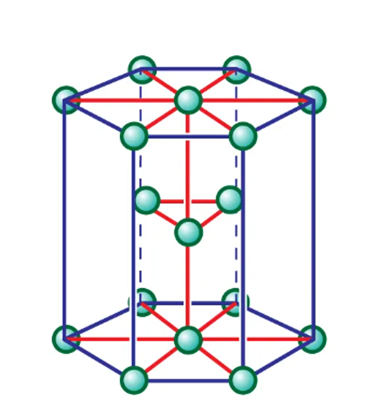
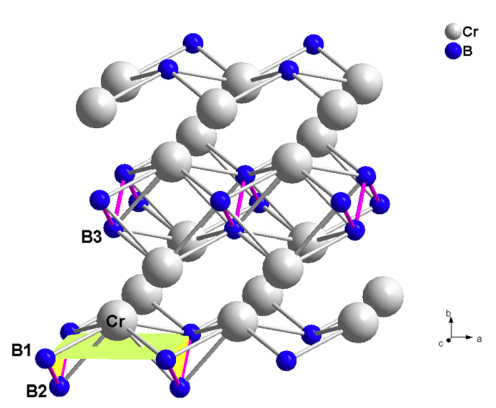
Crystal structure
Niobium boride has a crystal structure of hexagonal.
Properties and uses
Niobium boride is an ultra-high temperature ceramic (UHTC) with a melting point of 3050 °C. This along with its relatively low density of ~6.97 g/cm3 and good high-temperature strength makes it a candidate for high-temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities (Electrical resistivity of 25.7 µΩ⋅cm, CTE of 7.7⋅10−6 °C−1).
Niobium boride parts are usually hot pressed or spark plasma sintering(mechanical pressure applied to the heated powder) and then machined to shape. Sintering of Niobium boride is hindered by the material's covalent nature and the presence of surface oxides which increase grain coarsening before densification during sintering.
Synthesis
Niobium boride can be synthesized by the stoichiometric reaction between constituent elements, in this case, Nb and B. This reaction provides for precise stoichiometric control of the materials. Reduction of Nb2O5 (or NbO2) to niobium diboride can also be achieved via metallothermic reduction. Inexpensive precursor materials are used and reacted according to the reaction below:
Nb2O5 + 2 B2O3 + 11 Mg → 2 NbB2 + 11 MgO