Crystal Structure and Superconductivity of Vanadium Silicide
In the last sixty years, A15 intermetallic compounds with the formula A3B (where A is a transition metal and B is any element) have been very actively studied since many of these compounds display superconductivity at relatively high temperatures. Interestingly, some, such as V3Si (vanadium silicide), Nb3Sn and Nb3Al display the best combination of critical current density, superconducting temperature and critical magnetic field strength[1].
Superconductivity
These are important Type II superconductors, with applications of high critical currents for high magnetic field operations. For example, these A15 superconductors can be largely utilized in nuclear magnetic resonance spectroscopy and nuclear fusion reactions. Despite being the first discovered A15 superconductor, vanadium silicide has received remarkable experimental attention in recent years. In particular, this has been reported to be exhibit the superconducting transition temperature (Tc) of 17 K. With this value of Tc, the electron-phonon coupling parameter (λ) has been estimated to be 1.07, confirming that vanadium silicide is a strongly coupled BCS superconductor[1].
Crystal Structure
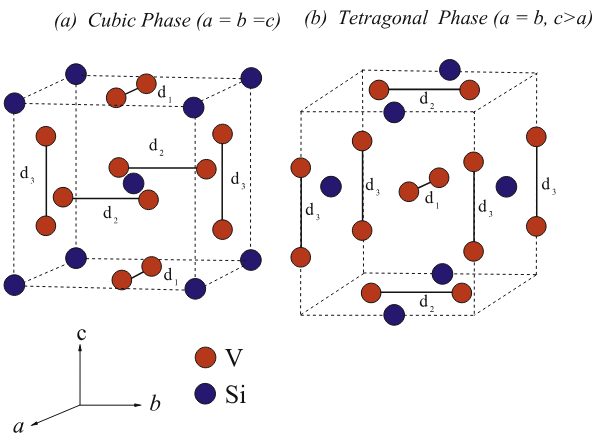
Figure 1. (a) The crystal structure of cubic V3Si. (b) The crystal structure of tetragonal V3Si. For both phases, V atoms form a system of three basically non-interacting orthogonal linear chains with the bond-lengths of d1, d2 and d3.[1]
The cubic phase of vanadium silicide, shown in Fig. 1 (a), is formed with two formula units per unit cell. This figure clearly shows that only one type of chemical bond between V atoms exists in this structure. Thus, we can conclude that the role of Si atoms is to stabilize the crystal structure of the cubic phase. In this structure, V atoms lengthen in pairs on the cube faces while Si atoms occupy a body-centered lattice (bcc).
Furthermore, V atoms generate a system of three basically non-interacting orthogonal linear chains with the bond-lengths of d1, d2 and d3 (see Fig. 1(a)). Due to the cubic symmetry, these bonds are equal in length, of value 2.3504 Å. It is worth mentioning that this value is shorter than the corresponding value of 2.3624 Å in the bcc metal V. This finding broadly reveals that the V-V bonding in the cubic phase of V3Si is stronger as compared to the corresponding bonding in the bcc metal V.
As mentioned before, the phase of vanadium silicide becomes tetragonal, with a very small deviation of c/a from unity, as shown in Fig. 1(b). The tetragonal phase of this material belongs to the space group P42/mmc. This phase also contains two formula units (six V and two Si atoms) in the unit cell with Wyckoff positions at V (2e) (0, 0, 1/4), V (4k) (1/4, 1/2, 1/2) and Si (2c) (0, 1/2, 0).
Reference
[1] The effect of martensitic phase transition from cubic to tetragonal on the physical properties of V3Si superconductor. doi:10.1016/j.intermet.2018.02.010