Crisaborole ointment: Indications, Safety and Efficacy
What is Crisaborole ointment?
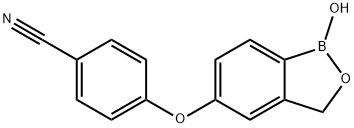
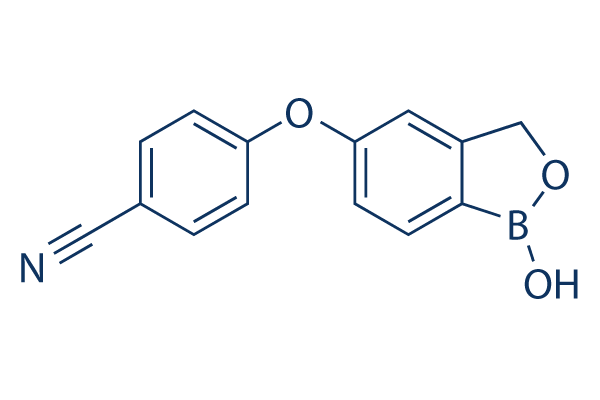
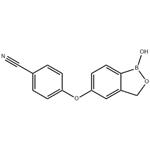
Crisaborole has broad-spectrum anti-inflammatory activity by mainly targeting phosphodiesterase 4 (PDE4) enzyme that is a key regulator of inflammatory cytokine production. As this enzyme is expressed in keratinocytes and immune cells, crisaborole mediates an anti-inflammatory effect on almost all inflammatory cells. Topical application of this drug is useful as it potentiates the localization of this drug in the skin and this anti-inflammatory activity is in the low micromolar range.
Crisaborole ointment (2%) is a steroid-free ointment for the treatment of adults and children with mild to moderate eczema, for topical use only. It is a non-steroidal topical anti-inflammatory phosphodiesterase-4 (PDE4) inhibitor, which exerts its therapeutic effect by inhibiting inflammatory signalling pathways and the release of associated pro-inflammatory mediators.
Indications
Crisaborole ointment (2%) is approved for the treatment of mild to moderate atopic dermatitis (AD) and is effective in relieving symptoms of skin inflammation, itching and rash. It is an alternative treatment to applying corticosteroids, pimecrolimus ointment, or tacrolimus ointment to the skin for eczema.
Safety and Efficacy
In a clinical trial of crisaborole, crisaborole ointment and placebo moisturising ointment were given to 1522 patients with mild to moderate eczema, ranging in age from 2 to 79 years. Results showed that about one-third of the patients treated with crisaborole ointment had clear or nearly clear skin and more had relief of itchy skin after applying the ointment twice daily for 28 days. At the end of the study, 57 per cent of patients using crisaborole no longer had itchy skin, compared to 40 per cent of patients using the moisturising cream.
In another clinical study of the efficacy and safety of crisaborole ointment in paediatric atopic dermatitis over a four-week period, 19 children between the ages of 2 and 16 years with mild to moderate AD used crisaborole ointment on the affected area twice daily for 30 days. The results showed that the children's mean ISGA score decreased from 2.58 ± 0.61 to 0.95 ± 0.78, indicating a significant reduction in AD severity, and the SPS score decreased from a mean value of 2.32 ± 0.478 to 0.84 ± 0.60 (p < 0.001), indicating a significant reduction in pruritus. In addition, there was a significant improvement in all indicators of clinical signs of AD, including erythema, exudation, peeling, induration/papules, and lichenification. Crisaborole ointment was well tolerated by patients.
References:
[1] PATEL S V. FORMULATION AND EVALUATION OF OINTMENT CONTAINING CRISABOROLE[J]. International Scientific Journal of Engineering and Management, 2023, 2 1: 45-51. DOI:10.55041/isjem01301.[2] DE A, CHAKRABORTY D, GRISILDA B N, et al. Efficacy and safety of crisaborole ointment in pediatric atopic dermatitis: A 4-week open-label study[J]. Indian Journal of Skin Allergy, 2024, 13 7: 2539-2916. DOI:10.25259/ijsa\_45\_2023.
You may like
Related articles And Qustion
See also
Lastest Price from Crisaborole manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $1.00/kg2025-07-08
- CAS:
- 906673-24-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000