Clomiphene citrate: applications in the treatment of infertility
Clomiphene citrate is an oral non-steroidal anti-estrogen that has the effect of stimulating ovulation. The pharmacologically active part of Clomiphene citrate is clomiphene. To increase the water solubility and oral bioavailability of the drug, clomiphene is usually produced in the form of clomiphene citrate. In 1967, Clomiphene citrate received FDA approval for marketing in the United States and was manufactured by Merrell-National (Laboratories) Ltd. and marketed under the name Clomid. Clomiphene is used to treat infertility in women who are not ovulating and is intended for women who have reached certain levels of estrogen in their bodies. Later, it was also used to treat male hypogonadism[1].

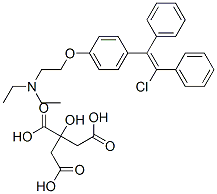
Figure 1. Clomiphene citrate
Pharmacology
Clomiphene citrate is primarily used to treat female infertility and male hypogonadism.
Polycystic Ovary Syndrome (PCOS) is a common metabolic disorder of reproductive endocrinology.The clinical features of PCOS include endocrine disorders, thinning of the hair, amenorrhoea, anovulation, hirsutism, and acne. PCOS not only leads to endocrine dysfunction in the body, but also under the influence of certain psychiatric disorders or medications, it leads to an imbalance in the ratio of gonadotropins and an inability to secrete progesterone, which can in turn lead to infertility. Infertility is caused by the inability to secrete progesterone. Since polycystic ovary syndrome is associated with insulin resistance and hyperinsulinaemia, the clinical management of PCOS consists mainly of medication and lifestyle changes. Da Vinci-35 and clomiphene citrate are commonly used clinical therapeutic agents[2].
Two brands of clomiphene citrate are sold in the United States and the other brand is sold in Canada. All three brands of clomiphene citrate are bioequivalent. Clomiphene citrate sold in both countries is a racemic mixture. Approximately 38% of the mixture is zuclomiphene (cis-isomer: formerly known as trans-clomiphene) and approximately 62% of the mixture is enclosmiphene (trans-isomer, formerly known as cis-clomiphene). Zuclomiphene citrate has mild estrogenic and anti-estrogenic effects, but enclosmiphene citrate is fully anti-estrogenic. Zuclomiphene induces ovulation approximately five times more than clomiphene citrate [3].
In 1967, clomiphene citrate was officially used clinically for the treatment of infertility. It revolutionised the treatment of infertility, particularly in polycystic ovary syndrome (PCOS). The original indication for clomiphene was the treatment of polycystic ovary syndrome and other anovulatory disorders in which ovarian activity can be demonstrated by luteinising hormone haemorrhage[3]. Clomiphene alone or in combination with human menopausal gonadotropin (HMG) and follicle-stimulating hormone (FSH) improves preovulatory follicle counts and luteal insufficiency in patients with unexplained infertility and in patients requiring artificial insemination (AI) by the husband or donor[4].
Mechanism of Action
Clomiphene citrate interacts with oestrogen receptors in many tissues, including the hypothalamus, pituitary, ovaries, endometrium, vagina and cervix. Clomiphene citrate inhibits the action of estrogen. The inhibition of oestrogen can lead to cause the pituitary gland to release follicle stimulating hormone (fSH), which promotes follicular growth and maturation, follicular rupture and ovulation.
Clomiphene citrate is a competitive antagonist of 17β-estradiol in the cytoplasmic nuclear receptor complex and other sites. It can cause an increase in gonadotropin-releasing hormone (GnRH) by blocking estrogen receptors in the hypothalamic arcuate nucleus. In addition, clomiphene can also increase the sensitivity of the pituitary gland to gonadotropin-releasing hormone, which has a similar effect to estradiol. During the administration of Clomiphene citrate, the secretion of FSH and LH increases. Clomiphene citrate also has a direct effect on the ovaries, making granulosa cells more sensitive to pituitary gonadotropin[5]. During the luteal phase of the cycle, serum concentrations of progesterone and estradiol increase, and there is a direct dose-response relationship between the two[6]. The increase in estrogen concentration continued until the 16th week of gestation, and the increase in progesterone concentration continued until the 11th week after ovulation, and then returned to the same value as in natural pregnancy. Increased concentrations of estradiol and progesterone led to an increase in uterine artery diameter and a modest increase in uterine artery volume flow, but the resistance index (RI) remained unchanged during the first 9 weeks of pregnancy[6]. All of these changes are associated with Clomiphene citrate[6].
Absorption, Distribution, Metabolism, and Excretion
Clomiphene citrate can be taken orally. After 5 days of oral administration, 51% of Clomiphene citrate is excreted, but some Clomiphene citrate continues to be excreted for at least 6 weeks. Plasma concentrations peaked 6 hours after oral administration of 50 mg of levamiphene. Plasma concentrations reached a stable state of 25% of the peak after 48 hours and remained stable for the next 14 days[7].
Toxic and Side Effects
When Clomiphene citrate is taken at the recommended dose, side effects are not serious and treatment is not interrupted. Side effects include hot flashes, bloating, occasional abdominal pain, enlarged ovaries, blurred vision and, less frequently nausea and vomiting. Close monitoring must be maintained throughout the treatment process and any abnormal symptoms must be investigated[8].
Synthesis
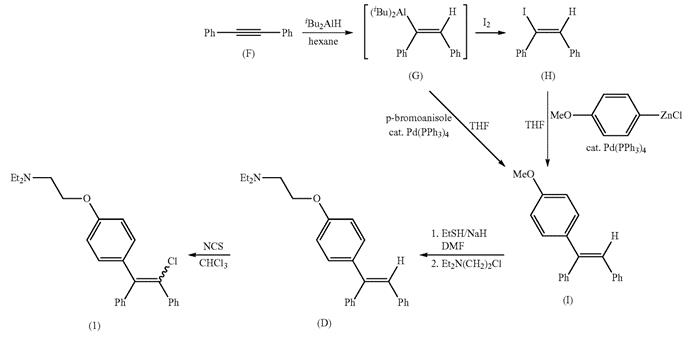
Figure 2. Clomiphene citrate synthesis
Clomiphene is prepared by hydroamination of diphenylacetylene (F) followed by palladium-catalyzed cross-coupling. In this process, diphenylacetylene (F) is hydrogenated and ammoniated to obtain vinylpropane (G). G can be used directly or by iodine cracking to obtain iodized vinyl (FI). Subsequently, vinylpropane (G) is cross-linked with p-bromoanisole or iodized vinyl (Fl) and (p-methoxyphenyl) zinc chloride to give the methoxyaryl compound (I). (I) is demethylated with sodium ethanethiolate, then the resulting phenyl oxide is alkylated with 2-(N, n-diethylaminoethyl) ethyl chloride to obtain triarylvinyl (D). Triarylvinyl (D) is chlorinated with n-chlorosuccinimide to obtain clomiphene (1).
Conclusions
Clomiphene citrate has strong antiestrogenic activity and weak estrogenic activity. Small doses of Clomiphene citrate can promote the secretion of gonadotropin from the anterior pituitary gland, thus inducing ovulation. Large doses of Clomiphene citrate can significantly inhibit pituitary release of gonadotropin, and then promote male spermatogenesis, which is effective for oligospermia. Although the drug has been on the market for decades, the mechanism by which it stimulates ovulation is not fully understood. At present, the more reliable explanation is that Clomiphene citrate has weak excitatory effect and strong antagonistic effect on estrogen. Stimulation of ovulation may be done in the hypothalamus. Although the mechanism of action of Clomiphene citrate is not fully understood and there are many side effects, due to its excellent pharmacological activity, Clomiphene citrate is still the drug of choice for anovulatory women and ovulatory women with follicular or luteal dysfunction.
Reference
[1]Patel, Dineshkumar; Karadeolian, Avedis; Souza, Fabio E. S.; Rey, Allan W.United States, US20220119331 A1 2022-04-21
[2]Huijben M, Lock MTWT, de Kemp VF, de Kort LMO, van Breda HMK. Clomiphene citrate for men with hypogonadism: a systematic review and meta-analysis. Andrology. 2022;10(3):451-469. doi:10.1111/andr.13146
[3]Collée J, Mawet M, Tebache L, Nisolle M, Brichant G. Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments. Gynecol Endocrinol. 2021;37(10):869-874. doi:10.1080/09513590.2021.1958310
[4]Clomiphene. In:Drugs and Lactation Database (LactMed?). Bethesda (MD): National Institute of Child Health and Human Development; September 20, 2021.
[5]Dickey RP, Holtkamp DE. Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update. 1996;2(6):483-506. doi:10.1093/humupd/2.6.483
[6]Glasier AF. Clomiphene citrate. Baillieres Clin Obstet Gynaecol. 1990;4(3):491-501. doi:10.1016/s0950-3552(05)80306-2
[7]Davidson R, Motan T, Korownyk C. Clomiphene for anovulatory infertility. Can Fam Physician. 2016;62(6):492.
[8]Rozen T. Clomiphene citrate for treatment refractory chronic cluster headache. Headache. 2008;48(2):286-290. doi:10.1111/j.1526-4610.2007.00995.x
You may like
Related articles And Qustion
See also
Lastest Price from Clomiphene Citrate manufacturers

US $0.00/Box2025-09-26
- CAS:
- 50-41-9
- Min. Order:
- 1Box
- Purity:
- 0.99
- Supply Ability:
- 10tons

US $0.00/Box2025-09-26
- CAS:
- 50-41-9
- Min. Order:
- 1Box
- Purity:
- 0.99
- Supply Ability:
- 10tons