Chrysophanic acid: Biosynthesis and chemical synthesis
Description
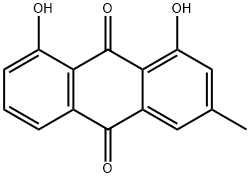
Chrysophanol is an anthracene derivative with two ketone groups attached to the central benzene ring. It is also known as chrysophanic acid. The molecular formula of chrysophanol is C15H10O4, the molecular weight is 254.2 g/mol, and the melting point is 196 °C. It is a crystalline solid, occurring as a golden yellow or brown powder, and exhibits maximum UV absorption at 225, 257, 277, 287, and 428 nm. The solubility of chrysophanol in water is poor; the aqueous solution is yellow but turns red with the addition of an alkali or concentrated sulfuric acid. The chrysophanol possesses two hydrogen bond donors and four hydrogen bond acceptors. The log P value for chrysophanol is 2.810. Low lipophilicity and low log P values enhance the absorption or permeation of the drug. Conclusively, the chemical properties of chrysophanol recommend that it is suitable as an orally active drug[1].
Discovery
Chrysophanic acid is obtained from "Goa powder," which is found in the heartwood or cavities formed by decay in the wood of "Andira Araroba," a tree found in Brazil. It is also found in Rhein, U. S. P., and is produced by the oxidation of Chrysarobinum, U. S. P., a constituent of Goa powder. Lieberman proved that the latter's acid was largely present and easily transformed by oxidation. It is a pale orange-yellow microcrystalline powder, odorless and tasteless, and which, on exposure to air, turns a brownish-yellow color.
Biosynthesis
Chrysophanol is naturally synthesized via the PMA or octaketide pathway; eight units of acetyl CoA are condensed by polyketide synthase. Leistner and Zenk reported that chrysophanol is synthesized in fungi through the PMA pathway, whereas in plants, it is synthesized through both shikimate and PMA pathways. The cyclization or folding pattern of the octaketide chain is different in different organisms. The occurrence of two folding patterns in nature is well established; these are categorized as "F" mode, named because of its occurrence in fungus, and "S" mode, observed in the bacterial strain Streptomyces. In plants, insects, and fungi, the polyketide chain is cyclized through "F" mode, in which the first ring of polyketide is built by two C2 units (acetyl CoA), whereas in "S" mode, it is formed by three C2 units. The first and middle rings of chrysophanol are synthesized via polyketide chain cyclization, and the last ring, having a methyl group, is formed through decarboxylation after aldol reaction within intermediate molecules. Emodin and emodinanthrone can form chrysophanol and chrysophanol anthrone by dehydroxylation in the presence of the dehydrolase enzyme. Further oxidation of chrysophanol anthrone yields chrysophanol[2].
Chemical synthesis
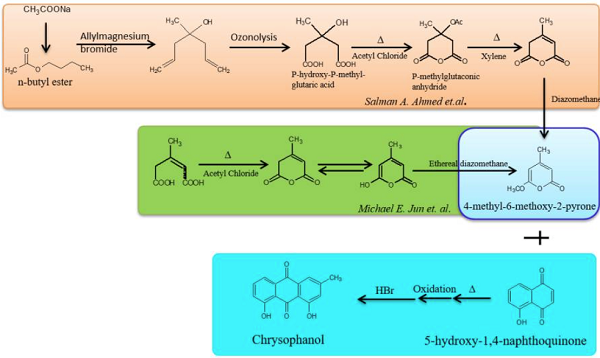
Several researchers have attempted to chemically synthesize chrysophanol. Two methods using Friedel-Craft and Diels-Alder reactions have been mainly studied; in both these reactions, a common pyrone derivative, 4-methyl-6-methoxy-2-pyrone, is synthesized. This pyrone derivative, when heated with juglone (5-hydroxy-1,4-naphthoquinone) and hydrolyzed after oxidation, was shown to produce chrysophanol at a yield of 62%. The synthesis of 4-methyl-6-methoxy-2-pyrone was found to differ in both methods. The Diels-Alder reaction has an advantage over the Friedel-Crafts reaction in which pyrone might be converted into 2,6-dioxygenated pyrylium salt in solid electrophiles or Lewis acids. A convenient synthetic procedure of synthesizing pyrone was proposed in which the reaction was started with sodium acetate, and ultimately, 4-methyl-6-methoxy-2-pyrone was synthesized after seven steps and was finally converted to chrysophanol. However, all the methods of synthesis yield the same amount of chrysophanol (62% of all the reactants), and efforts to increase the yield have yet to be successful.
References
[1] Fox, C. “THERAPEUTIC VALUE OF CHRYSOPHANIC ACID IN DERMATOLOGY.” 1906. 0.
[2] Mohd Aslam Yusuf. “Chrysophanol: A Natural Anthraquinone with Multifaceted Biotherapeutic Potential.” Biomolecules (2019).
You may like
See also
Lastest Price from Chrysophanic acid manufacturers

US $0.00/kg2025-05-07
- CAS:
- 481-74-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $150.00/kg2025-04-21
- CAS:
- 481-74-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg