Chlorinated polypropylene: Physicochemical property, Structure and Application
Physicochemical property
The Chlorinated polypropylene (CPP) density was 0.93 g/mL at 25℃. It is soluble in methylene chloride, toluene and tetrahydrofuran.
Densities and viscosities
The densities for binary mixtures of fractionated chlorinated polypropylene with toluene and 2- butanone have been determined. At a fixed temperature, concentration, and solvent, the densities for binary mixtures of fractionated and unfractionated chlorinated polypropylene with solvents are the same. The width of the molecular weight distribution of chlorinated polypropylene does not affect the densities of these mixtures. The densities and viscosities for binary mixtures of chlorinated polypropylene with toluene, tetrahydrofuran, chloroform, carbon tetrachloride, and 2- butanone have been measured at temperatures from ( 298.15 to 318.15) K. The apparent molar volumes and standard partial molar volumes of the chlorinated polypropylene repeat unit were calculated from experimental measurements. From the results, the repeat unit structure of chlorinated polypropylene has been determined[1].
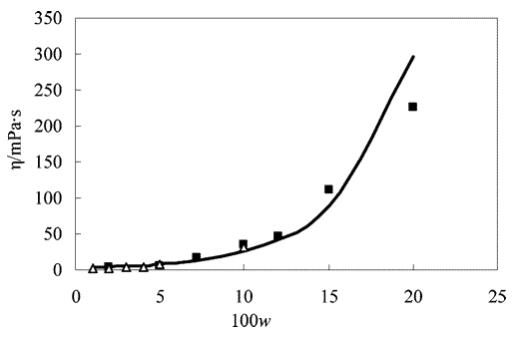
Figure 1 Comparison of viscosity of CPP + toluene mixtures with literature:? ?, experimental data; ?, literature data; ——, fitting curve.
Kinetic study
Acrylic acid (AA) grafting onto chlorinated polypropylene (CPP) was carried out using toluene as solvent and benzoyl peroxide (BPO) as initiator by free radical polymerization. The effects of reaction temperature, concentration of acrylic acid monomer and benzoyl peroxide initiator on the rate of graft polymerization were studied. The rate of graft polymerization depended on the initiator and monomer concentrations. The actual rate constant of graft polymerization was found to obey the Arrhenius law and the activation energy E value was 40.6 kJ/mol. The kinetic equation for the grafting of acrylic acid onto chlorinated polypropylene was R = K [BPO] [AA](0.62) [2].
Structure
Study on the microstructure
The microstructure of chlorinated polypropylene is studied by means of C-13-NMR method. All the possible structures in chlorinated polypropylene are analyzed and chemical shifts of carbons in the possible structures are calculated according to the literature. From the comparison between experimental and calculated chemical shifts, each absorption peak in the spectra is attributed to some special carbon. The information of chlorine atoms distributing on the chain of chlorinated polypropylene produced by different technical process can give some good suggestions for improving the preparation process of chlorinated polypropylene [3].
Structural-Analysis
Experimental splitting of the C-13 NMR spectrum of chlorinated polypropylene has been investigated, because the spectra were very intricate and the peaks widely overlapped with each other. Since the DEPT (distortionless enhancement by polarization transfer) spectra were still insufficient in separation of the overlapped peaks,separation between the signals of chlorinated carbon and that of nonchlorinated carbon were examined. For this purpose, the pulse sequence for INEPT (insensitive nuclei enhanced by polarization transfer) was found to be suitable when the refocusing delay (D3) was set to either 1/J(CH) of chlorinated or nonchlorinated carbon. Then, the signals of the carbon corresponding to the J(CH) value disappeared but residual carbon signals maintained adequate strength for analysis. Addition and/or subtraction of the DEPT and the INEPT spectra gave the subspectra classified according to the attached proton number and the attached chlorine number. That is the individual subspertra of RCH3 (R = alkyl), RCH2Cl, R2CH2 R3CH, R2CHCl, and RCHCl2 were able to be edited [4].
Application
Used for preparing acrylamide containing TPR surface primer
Thermoplastic elastomer (also known as thermoplastic rubber), which is mainly made of symmetrical block styrene butadiene rubber (SBS rubber) and is processed with other materials, is a polymer material that combines the characteristics of rubber and thermoplastic plastics, shows high elasticity of rubber at room temperature, and can be plasticized at high temperature. It is also the so-called third generation rubber after natural rubber and synthetic rubber, abbreviated as TPR. Because of its easy processing and low price, it is widely used as sole material, especially for middle and high grade sports shoes. However, due to its weak polarity, in the cold adhesive shoe industry, in order to improve the adhesive strength, TPR adhesive materials often need to be surface treated. The surface treatment method is generally grinding wheel to make it have rough surface, or using surface treatment agent to change the surface state of sole materials, or both at the same time to achieve good bonding effect. The toluene solution of trichloroisocyanuric acid can play a good bonding effect after treating TPR, but it is unstable and needs to be prepared temporarily, and it is easy to make the surface of the material yellow and affect the appearance. The preparation method is as follows: first, prepare SBS graft acrylamide copolymer lotion and chlorinated polypropylene graft copolymer lotion respectively, then mix 100 parts of SBS graft acrylamide copolymer lotion, 20-80 parts of chlorinated polypropylene graft copolymer lotion and 10-15 parts of polyvinylpyrrolidone evenly to obtain the water-borne surface treatment agent of TPR sole material [5].
Effects on the bonding performance of an epoxy core rod and polyolefin sheath for composite insulators
One of the key technologies of composite insulators is the bonding between the core rod and sheath. This paper explores the use of chlorinated polypropylene-based adhesives to bond a polyolefin sheath material and an epoxy core rod. Dye penetration tests, bonding strength tests and water diffusion tests were carried out to evaluate the adhesiveness. The results demonstrated that the sample bonded by the chlorinated polypropylene/gamma-methacryloxypropyltrimethoxysilane adhesive had the best interface tightness, and the bonding strength reached 7.34 MPa. Furthermore, the leakage current was as low as 41 mu A after the sample was placed in boiling water for 100 h. This work provides a key bonding technology for polyolefin materials used as composite insulator sheaths [6].
Promoting printability of biaxially oriented polypropylene film
In this paper, a polymeric coating based on the modified chlorinated polypropylene (CPP) emulsion was synthesized, methyl methacrylate (MMA), butyl acrylate (BA) and acrylic acid (AA) were grafted onto CPP backbone and phase inversion was conducted to obtain waterborne emulsion. Results showed that the concentration of initiator (BPO) had the greatest effect on graft copolymerization. The concentration of emulsifier and temperature influenced the results of phase inversion. Besides, the thermal performances of modified CPP were better than untreated one. In addition, the coating obtained in optimum condition had excellent adhesion to BOPP film, and apparently improved the printing quality of the film. The printability promotion should be attributed to the different movement trend of coating's polar and un-polar chains during the baking step, as well as the subsequent formations of new coating/substrate and coating/ink interface layer [7].
Adhesion properties its adhesion mechanism for untreated polypropylene materials
The adhesion and coa
You may like
Related articles And Qustion

US $10.00/kg2025-04-21
- CAS:
- 68442-33-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $180.00/kg2025-04-15
- CAS:
- 68442-33-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton