Chloramphenicol: Chemistry, Mechanism of Action and Pharmacokinetics
General Description
Chloramphenicol is a broad-spectrum antibiotic that can be taken orally or topically with minimal harmful effects. It is an Amphenicol-class Antibacterial. Initially, systemic (oral and intravenous) chloramphenicol had positive results against Rickettsia, the causative agent of typhus. In one trial, 25 confirmed cases of scrub typhus were successfully treated with chloramphenicol, with no reports of mortality or complications. Currently, chloramphenicol is available in various forms, including oral tablets, oral liquid suspension, intravenous solution, ointment, and liquid eye drops. Due to its broad spectrum of activity and apparent safety, it is widely used as a topical treatment for ocular infections.
However, in rare cases and in certain susceptible populations, systemic chloramphenicol has been associated with blood dyscrasias, including aplastic anemia, and has a higher risk. Despite its fall from favor as a systemic treatment option, chloramphenicol remains the most commonly used topical antibiotic for ocular surgical prophylaxis and superficial ocular infections in the United States. The British National Pharmacopoeia also supports the use of chloramphenicol as the drug of choice for superficial ocular infections”. Incredibly, there has been little resistance to topical chloramphenicol, and some speculate that this is due to limited systemic use. Interestingly, systemic chloramphenicol is now being used again, and the British National Pharmacopoeia recommends its use for invasive Haemophilus influenzae and Rickettsia infections, whereas tetracyclines are contraindicated. The use of systemic chloramphenicol should be limited to potentially life-threatening infections.3

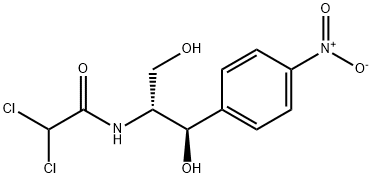
Figure 1. Chloramphenicol
Chemistry
Chloramphenicol is a broad-spectrum antibiotic that was originally isolated from the soil organism Streptomyces venezuelae. It is notable for being one of the first antibiotics to be manufactured synthetically on a large scale. Chloramphenicol is available in various forms, including oral capsules and injectable solutions. In the United States, the parenteral form, chloromycetin sodium succinate, continues to be available, albeit in limited quantities. The oral form, chloromycetin palmitate, while no longer produced in the U.S., remains accessible in other parts of the world. This accessibility underscores the continued relevance of chloramphenicol in global healthcare, particularly in areas where newer antibiotics might not be available or affordable. 1
Mechanism of Action
Chloramphenicol binds to the 50 S 70 S ribosome Subunit binding inhibits the action of peptidyl transferase, thereby preventing the formation of peptide bonds and, in turn, inhibiting the synthesis of microbial proteins. This mechanism also prevents aminoacyl transfer RNA from binding to the active site of peptidyl transferase. 1
Pharmacokinetics
There are three most commonly used chloramphenicol preparations in clinical practice: oral crystalline powder, palmitate ester for oral suspension, and succinate ester for parenteral administration. Both esters are inactive and require hydrolysis to chloramphenicol to exert their antimicrobial effects. Palmitate ester is hydrolyzed to active chloramphenicol in the small intestine and then absorbed. Chloramphenicol succinate is a prodrug that is converted to active chloramphenicol while circulating in the body. The bioavailability of oral crystalline chloramphenicol and chloramphenicol palmitate is approximately 80%. The time to peak plasma concentration depends on particle size and is related to the in vitro dissolution and depolymerization rates. The bioavailability of chloramphenicol after intravenous administration of the succinate ester averages approximately 70%, but the range is quite variable. Incomplete bioavailability is due to the excretion of unchanged chloramphenicol succinate through the kidneys before it is hydrolyzed to active chloramphenicol. The plasma protein binding rate of chloramphenicol in healthy adults is approximately 60%. The drug is widely distributed in many tissues and fluids, including cerebrospinal fluid and breast milk, and crosses the placenta. Reported mean apparent volumes of distribution range from 0.6 to 1.0 L/kg. Most of the chloramphenicol dose is metabolized by the liver to inactive products, with the major metabolite being the glucuronide conjugate; only 5% to 15% of chloramphenicol is excreted unchanged in the urine. The elimination half-life is approximately 4 hours. 2,4
References:
[1] BALBI H J. Chloramphenicol: a review.[J]. Pediatrics in review, 2004, 25 8. DOI:10.1542/pir.25-8-284.You may like
Related articles And Qustion
Lastest Price from Chloramphenicol manufacturers

US $0.00/kg2025-10-09
- CAS:
- 56-75-7
- Min. Order:
- 25kg
- Purity:
- 97.5%-102%,BP2024
- Supply Ability:
- 100MT
US $0.00-0.00/kg2025-06-03
- CAS:
- 56-75-7
- Min. Order:
- 1kg
- Purity:
- 98.0%-102.0%; BP/EP
- Supply Ability:
- 500KG