The utility of chloramphenicol
Introduction
The discovery of chloramphenicol was first announced by Ehrlich et al. and independently by a group at the University of Illinois. Preliminary chemical properties were described by Bartz, and a complete structural determination was reported by Rebstock et al.. This antibiotic is unique in that it was the first natural product found that contained a nitro group and also the first natural product which was a derivative of dichloroacetic acid[1].
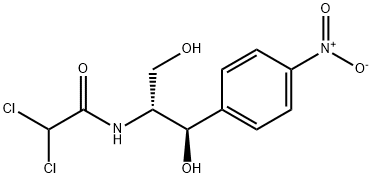
Several generic names have been used for chloramphenicol. The preferred Chemical Abstracts name is D-threo-2 ,2-dichloro-N-[3-hydroxy-a-(hy[1]droxymethyl)-p-nitrophenethyl]-acetamide, but the name more commonly seen in the literature is D(-) threo-2-dichloroacetamido-1-p-nitrophenyl[1]1,3-propanediol. It can be seen that there are two asymmetric carbon atoms, leading to four possible stereoisomers. All four isomers have been synthesized, and the two erythro isomers are biologically inactive, whereas the L(+) threo isomer has less than 0.5 per cent of the activity of the natural D(-) threo isomer.

Picture 1 Chloramphenicol powders
The clinically useful antibiotics
Chloramphenicol was the first of the clinically useful antibiotics to be synthesized and the only one which is marketed in synthetic form today. Because of its relatively simple structure, a large number of modifications of this antibiotic have been prepared and tested. A number of biochemists and physiologists have viewed its simple structure and have been encouraged to work on it, possibly with the feeling that what looks simple must have a simple mode of action. This view has proved to be erroneous, but has led to a large number of interesting studies. In recent years chloramphenicol has become a tool of molecular biologists and geneticists working on the synthesis and function of nucleic acids, since it can inhibit protein synthesis while al[1]lowing continued nucleic acid synthesis.
The utility of chloramphenicol
Because of the great clinical utility of chloramphenicol and its relative ease of chemical synthesis, it was natural that a large number of chloramphenicol analogues would be made and tested for antibacterial activity. Although no analogue has proved superior to the natural antibiotic, the results of these studies have revealed some of the structural requirements for antibacterial activity in the chloramphenicol series. The relative activities of the four isomers have been compared by Maxwell and Nickel (76); only the D(-) threo isomer showed any significant activity. However, Hahn et al. (54) have shown that the synthesis of a D-glutamic acid poly[1]peptide by Bacillus subtilis is not inhibited by the D(-) threo isomer, but is inhibited by the L(+) erythro isomer. Since both these isomers have the OH group on carbon 1 in the same position, it is presumed that the stereochemical configuration at carbon 1 is essential for any biological activity, whereas the configuration at carbon 2 determines whether L-polypeptide (protein) or D-polypeptide synthesis will be inhibited. The structure of the propanediol moiety is critical for activity. If either hydroxyl is replaced by hydrogen atoms, all activity is lost, and the same is true if the hydroxyl groups are esterified. The propane chain cannot be extended, as in 1 ,3-diol-3-dimethylpropane or in butane-1,3- diol, without loss of activity. In addition, substitution of a methyl group for the hydrogen atom on carbon 2 leads to a loss of activity. In the acetamide side chain, the size of the constituent and its electronegativity influence the activity, but there is no absolute requirement for the chlorine atoms. Also, the free base of chloramphenicol, resulting from the complete removal of the dichloroacetamide side chain, has 1.8 per cent of the activity of the parent drug. Further, if the free hydrogen on the nitrogen atom is replaced with a methyl group, all activity is lost.
Chloramphenicol is effective in inhibiting a wide variety of bacteria, from practically all families, at concentrations between 1 and 10 .jug per ml (77, 96). This is in contrast to penicillin, which inhibits all bacteria, but at widely varying concentrations, so that some species may be inhibited by 0.001 ug per ml, whereas others require 1000 /Ag per ml. Very few bacterial species are completely inhibited by concentrations of chloramphenicol less than 1 ,ug per ml. The spirochetes and the filamentous bacteria are also inhibited by chloramphenicol. Some groups such as the myxobacteria have apparently never been tested. There have been con- -flicting reports on the sensitivity of the pleuropneumonia-like organisms (genus Mycoplasma), since some workers have found them resistant, whereas others have found them to be sensitive. The "killer" particle in Paramecium aurelia, which may be a small obligately parasitic bacterium, is selectively inhibited by chloramphenicol. Viruses and rickettsias which are affected by tchloramphenicol include: lymphogranuloma, psittacosis, epidemic typhus, murine typhus, scrub -typhus, rickettsialpox, Rocky Mountain spotted fever, and Q fever. Those viruses which are unaffected by this antibiotic include: vaccinia, variola, St. Louis encephalitis, Japanese encephalitis, rabies, polio, Theiler's intestinal virus, mumps, influenza, distemper, Newcastle disease, chick bronchitis, and laryngotracheitis. The bacterial viruses are apparently not affected directly, but their growth is inhibited indirectly through effects on the metabolism of the host
Reference
1 Brock T D. Chloramphenicol[J]. Bacteriological Reviews, 1961, 25(1): 32-48.
Related articles And Qustion
Lastest Price from Chloramphenicol manufacturers

US $0.00/kg2025-10-09
- CAS:
- 56-75-7
- Min. Order:
- 25kg
- Purity:
- 97.5%-102%,BP2024
- Supply Ability:
- 100MT
US $0.00-0.00/kg2025-06-03
- CAS:
- 56-75-7
- Min. Order:
- 1kg
- Purity:
- 98.0%-102.0%; BP/EP
- Supply Ability:
- 500KG