Chloral hydrate:a sedative-hypnotic drug
Introduction
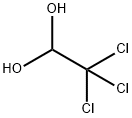
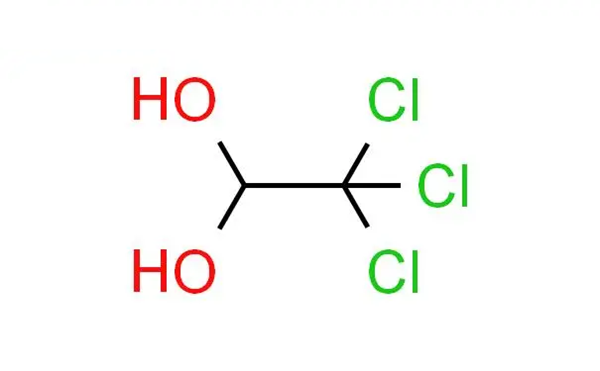
Chloral hydrate, a trichloroacetaldehyde monohydrate with the chemical formula C2H3Cl3O2, is a sedative-hypnotic drug that historically found application in insomnia treatment and anesthesia induction2. With roots dating back to the 19th century, it acts as a central nervous system depressant, potentiating the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) in the brain. However, its usage has significantly diminished in contemporary medicine due to the development of safer alternatives. While chloral hydrate was once commonly prescribed for its sedative effects, it is associated with various side effects, including drowsiness, dizziness, upset stomach, and headaches. Prolonged use or high doses can result in more severe complications, prompting a decrease in its medical use. In modern healthcare, chloral hydrate is not the primary choice for insomnia or sedation, and its availability is limited in many regions. Moreover, the potential for toxicity, marked by respiratory depression, cardiovascular collapse, and other adverse effects, underscores the need for caution. As a controlled substance in numerous jurisdictions, its regulation emphasizes the importance of careful administration and monitoring. Individuals considering sedative-hypnotic treatments should consult healthcare professionals to explore safer and more contemporary options tailored to their specific needs.
Application
Chloral hydrate has historically found application in the medical field for the treatment of insomnia and as an anesthetic agent. However, its use has significantly diminished over time due to the emergence of safer and more effective alternatives. Here are the key applications: Insomnia Treatment: Chloral hydrate was widely used in the past as a sedative to address insomnia. Its ability to induce sleep made it a common choice for individuals struggling with sleep disorders. Anesthesia: In the early days of anesthesia, chloral hydrate was utilized as an anesthetic agent, particularly for minor surgical procedures. Its sedative properties were beneficial in rendering patients unconscious during medical interventions. Sedation: Beyond its role in insomnia treatment, chloral hydrate was employed for sedation in various medical settings. This included calming effects for agitated patients or those undergoing certain diagnostic procedures. Despite these historical applications, chloral hydrate is no longer the preferred choice in contemporary medicine. Safer and more targeted medications with fewer side effects have largely replaced its use. The potential for toxicity and adverse effects, coupled with advancements in medical science, has led to a decline in the application of chloral hydrate in modern healthcare practices.
1. Sedation is often required for children undergoing diagnostic procedures. Chloral hydrate has been one of the sedative drugs most used in children over the last 3 decades, with supporting evidence for its efficacy and safety. Recently, chloral hydrate was banned in Italy and France, in consideration of evidence of its carcinogenicity and genotoxicity. Dexmedetomidine is a sedative with unique properties that has been increasingly used for procedural sedation in children. Several studies demonstrated its efficacy and safety for sedation in non-painful diagnostic procedures. Dexmedetomidine's impact on respiratory drive and airway patency and tone is much less when compared to the majority of other sedative agents. Administration via the intranasal route allows satisfactory procedural success rates. Studies that specifically compared intranasal dexmedetomidine and chloral hydrate for children undergoing non-painful procedures showed that dexmedetomidine was as effective as and safer than chloral hydrate. For these reasons, we suggest that intranasal dexmedetomidine could be a suitable alternative to chloral hydrate1.
2. Chloral hydrate, discovered in 1832, is one of the oldest sedatives in the market that has fallen in to disrepute at the beginning of this century in parts of Europe2. Although, used for sedating children in North America for several years, interest for using it as a sedative was rekindled after a systematic review of chloral hydrate sedations of pediatric patients in the emergency department was published in 2008 by the American College of Emergency Physicianschloral hydrate is safe and effective for sedation during dental treatments for children with mild asthma. Chloral hydrate is well absorbed orally and rectally, is rapidly metabolized by alcohol dehydrogenase in the liver and erythrocytes to trichloroethanol, its active metabolite and is often used to produce immobility for nonpainful diagnostic procedures such as radiological procedures, echocardiographies, and electroencephalograms, in infants and young children It evaluated the safety and efficacy of chloral hydrate by measuring changes in heart rate (HR), transcutaneous oxygen saturation, (SpO2), asthma score, behaviour, types and frequency of adverse reactions associated with chloral hydrate were assessed throughout treatment. Due to ineffective sedation and mild respiratory depression associated with chloral hydrate, newer, easily titrated medications, such as midazolam, may offer advantages3.
Synthesis
The synthesis of chloral hydrate involves several chemical steps that It is typically produced through the reaction of chloral (trichloroacetaldehyde) with water. Here is a simplified overview of the synthesis process: Chloral Production: The initial step involves the production of chloral. This can be achieved by the chlorination of acetaldehyde, a process that replaces some hydrogen atoms with chlorine atoms. The chemical reaction is as follows:
2 CH3CHO+Cl2→CCl3CHO+2 HCl2
Formation of Chloral Hydrate: Chloral is then combined with water to form chloral hydrate. This reaction is typically carried out in the presence of a catalyst such as sulfuric acid:
CCl3CHO+H2O→CCl3CH(OH)2
Safety
Chloral hydrate poses potential safety concerns, and its use is associated with several risks and side effects. Here are some key safety considerations: Toxicity: Chloral hydrate can be toxic, especially in higher doses. Overdose can lead to serious health complications, including respiratory depression, cardiovascular collapse, and even death. It is crucial to use chloral hydrate under the supervision of a qualified healthcare professional who can monitor dosage and response. Dependence and Abuse: Like many sedative-hypnotic drugs, chloral hydrate has the potential for abuse and dependence. Prolonged or inappropriate use may lead to the development of tolerance, dependence, and addiction. Side Effects: Common side effects of chloral hydrate include drowsiness, dizziness, upset stomach, headache, and skin rashes. These side effects can impair cognitive and motor functions, making activities such as driving unsafe. Interactions with Other Medications: Chloral hydrate may interact with other medications, including alcohol and drugs that depress the central nervous system. These interactions can potentiate the sedative effects and increase the risk of adverse reactions. Not Recommended for Long-Term Use: Due to its potential for toxicity and the availability of safer alternatives, chloral hydrate is generally not recommended for long-term use. It is typically reserved for short-term treatment of specific conditions, under careful medical supervision. Special Populations: Caution is advised when prescribing chloral hydrate to certain populations, such as elderly individuals or those with liver or kidney impairment, as they may be more susceptible to its effects. Regulatory Control: Chloral hydrate is often a regulated substance due to its potential for abuse and toxicity. Its availability may be restricted, and its use may be subject to legal and regulatory controls.
For example,chloral hydrate (CH) is a short-lived intermediate in the metabolism of trichloroethylene (TRI). TRI, CH, and two common metabolites, trichloroacetic acid (TCA) and dichloroacetic acid (DCA) have been shown to be hepatocarcinogenic in mice. To better understand the pharmacokinetics of these metabolites of TRI in humans, eight male volunteers, aged 24-39, were administered single doses of 500 or 1,500 mg or a series of three doses of 500 mg given at 48 h intervals, in three separate experiments. Given these safety considerations, individuals should not use chloral hydrate without proper medical supervision. If prescribed, it is essential to follow the prescribed dosage and duration, report any adverse effects to a healthcare provider, and avoid combining it with other substances without medical guidance. Always consult with a qualified healthcare professional for personalized advice and treatment options4.

Figure 1 General outline of chloral hydrate metabolism and the enterohepatic circulation of trichloroethanol–glucuronide (TCE–G) CH, chloral hydrate, TCE,trichloroethanol, TCA, trichloroacetate, and DCA, dichloroacetate. Note that the conversion of CH to TCE is reversible, while the conversion of CH to TCA is essentially irreversible.
Reference
1. Cozzi G, Norbedo S, Barbi E. Intranasal Dexmedetomidine for Procedural Sedation in Children, a Suitable Alternative to Chloral Hydrate. Paediatr Drugs. 2017 Apr;19(2):107-111.
2. Ratnapalan S. Chloral hydrate sedation in children. Clin Pediatr (Phila). 2014 Sep;53(10):933-6.
3. Abdulhamid I, Tremblay M, Stenger J, Tutag Lehr V. Chloral hydrate for sedation of children with asthma during dental treatment. Eur J Paediatr Dent. 2016 Jun;17(2):141-6.
4. Merdink JL, Robison LM, Stevens DK, Hu M, Parker JC, Bull RJ. Kinetics of chloral hydrate and its metabolites in male human volunteers. Toxicology. 2008 Mar 12;245(1-2):130-40.
You may like
Related articles And Qustion
See also
Lastest Price from Chloral hydrate manufacturers

US $10.00/ASSAYS2025-08-17
- CAS:
- 302-17-0
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $6.00/kg2025-04-21
- CAS:
- 302-17-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000