Chemical Properties of 2-Aminopyrimidine
2-Aminopyrimidine (2AP) is an aminopyrimidine carrying an amino group at position 2. A single molecule of 2-aminopyrimidine has two equivalent proton donors and two equivalent proton acceptors available for hydrogen bonding to neighbouring molecules. bond pattern of 2AP found in its crystal structure2 [shown as 2AP dimer]. Such a hydrogen bond pattern could be perturbed by addition of a guest that preferentially forms hydrogen bonds to 2AP rather than to itself. Carboxylic acids were chosen as possible guest molecules for cocrystallization with 2AP since they are better acids than 2AP and, like 2AP, they form cyclic hydrogen bonded dimers[1-3].
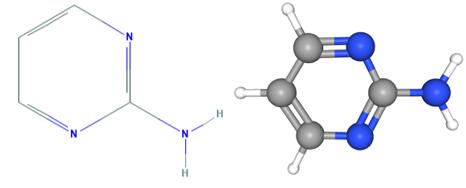
Fig 1. Chemical structure formula and three-dimensional structure of 2-Aminopyrimidine
The complexing ability of 2-aminopyrimidine derivatives with transition metal ions is of great interest since 2-aminopyrimidine has exo- and endo-cyclic nitrogen atoms for coordination and has ability to form a variety of molecules architectures through versatile coordination modes. Several transition metal complexes with 2-aminopyrimidine have been synthesized most frequently under hydrothermal conditions or anaerobic conditions[4,5].
2-Aminopyrimidine is a model compound for isocytosine (iC)–a building block of the nucleobase guanine (G) and some drugs such as folic acid (FA) and acyclovir (ACV). 2-Aminopyrimidine contains three functional groups, one exo NH2 group and two endo N-aza groups which are n–π and π–π conjugated with the endo >CC< group. One labile proton can move from the exo NH2 group to the endo N or C atom. Two types of the tautomeric conversions are possible: amine–imine tautomerism {NHC(R)N → NC(R)NH} from the exo to endo N atom or from one endo to the other endo N atom, and enamine–imine tautomerism {>CC(R)NH → >CHC(R)N} from the exo or endo N atom to the endo C atom[6].
Structure and magnetic studies of metal complexes with 2-aminopyrimidine (abbreviated as ampym) are of considerable interest due to the coordination diversity exhibited by the ligand and metal ions[7].
2-Aminopyrimidine has endo- and exo-cyclic nitrogen donors for coordination. The amino nitrogen atom is known to be more basic in comparison to the pyrimidine ring nitrogens. However, in some 2-aminopyrimidine complexes the coordination of the 2-aminopyrimidine with the metal occurs through the endocyclic ring nitrogen, whereas in others the 2-aminopyrimidine coordinates to the metal through the amino nitrogen.
References
[1] J. Scheinbein and E. Schempp, Acta Crystallogr., Sect. B, 1976,32.607.
[2] L. Leiserowitz, Acta Crystulfogr., Sect. B, 1976, 32, 775.
[3] An adenine m-bromobenzoic acid cocrystal has been reported which has a heterodimer interaction like the one proposed here. C.Tamura, N. Sakurai, and S. Sato, Bull. Chem. SOC. Jpn, 1971, 44,1473.
[4] Masoumeh Tabatabaee. (2-Aminopyrimidine-κN )diaqua(pyridine-2,6-dicarboxylato-κ O, N,O )nickel(II) monohydrate[J]. Acta Crystallographica, 2010, 66(Pt 6):m647-8.
[5] Jong-Ho Peter Lee, Blaine D. Lewis, Jessica M. Mendes. Transition metal halide salts and complexes of 2-aminopyrimidine: manganese(II) compounds – crystal structures of (2-aminopyrimidinium)4 [MnCl4(H2O)]2, [(2-aminopyrimidine)2MnBr2(H2O)2•2H2O and (2-aminopyrimidinium)2+[MnBr2(H2O)4]Br2[J]. Journal of Coordination Chemistry, 2003, 56(16):1425-1442.
See also
Lastest Price from 2-Aminopyrimidine manufacturers

US $0.00-0.00/KG2025-05-26
- CAS:
- 109-12-6
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $6.00/kg2025-04-21
- CAS:
- 109-12-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month