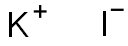Charge and formation of potassium iodide
Potassium iodide is an ionic compound that is made of one potassium and three iodine atoms.
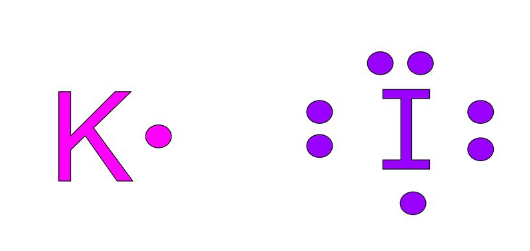
Charge and formation of potassium iodide
The chemical formula of potassium iodide is KI. Potassium has one valence shell electron and if it looses that, it will gain stabilty by loosing an electron.
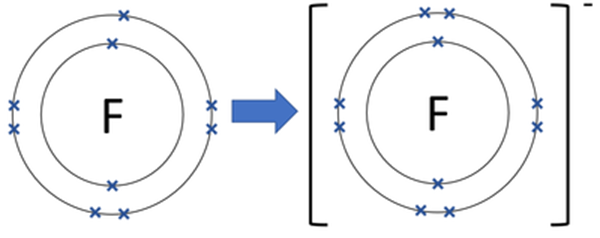
Iodine on the other hand has 7 electrons in the valence shell and needs one electron to attain stability.
Condition for stability- attainment of octet.
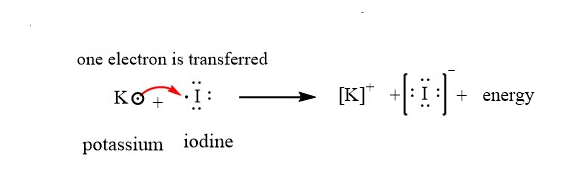
Therefore potassium loses one electron and becomes potassium ion.
This electron removed from potassium is gained by the iodine atom to form iodide anion.
The cation and anion are bonded through electrostatic forces of attraction between them and hence forms an ionic compound Potassium iodide.
Uses
Potassium iodide is used as an additive in regular table salt and acts as a nutritional supplement by decreasing the deficiency in iodine. It is also used in blocking radioactive iodine from entering the thyroid during nuclear disasters.
preparation
Potassium iodide (KI) is prepared by reacting potassium hydroxide to iodine with a hot solution. It is used mainly in the form of a saturated solution, 100 g potassium iodide to 100 ml water. That equates to around 50 mg/drop. Until drinking, the solution is normally applied to the tea, fruit juice or milk.
You may like
Related articles And Qustion
See also
Lastest Price from Potassium iodide manufacturers

US $10.00-1.00/kg2025-10-31
- CAS:
- 7681-11-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $1200.00-1100.00/ton2025-09-19
- CAS:
- 7681-11-0
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M