Formation of Fluoride Ions and Their Charges
How is the fluoride ion formed?
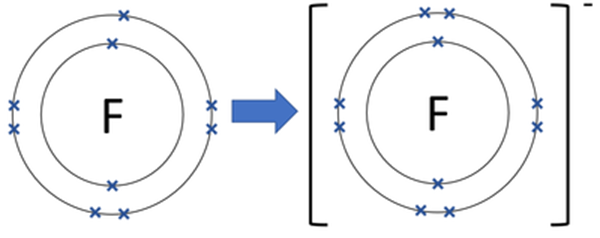
A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1. A fluoride ion is a negatively charged particle composed of a single fluorine atom. It is the simplest inorganic monoatomic anion of fluorine, which is considered a trace element. Fluoride ions exist in many minerals such as fluorite, apatite, and topaz. However, they are only present in trace amounts in water. The basic nature of ions permits them to create ionic bonds with atoms or molecules with positive charges.
What is the Charge of Fluorine Ion?
Fluoride ion has a charge of -1. To achieve the most stable electron configuration, a full octet, fluorine needs to gain one more electron. Fluorine completes the outermost shell by gaining an electron and becomes a negatively charged ion with a charge of 1. This charge allows it to form stable ionic compounds with other elements of opposite charges, such as metals. The formation of ions within elements is based on their electronic configuration and their tendency to achieve more stable configurations by gaining or losing electrons. Therefore, the charge of fluorine ion is -1.
Why does a Fluoride ion only have a -1 charge and not a -2 charge or more?
The octet rule is usually used to explain the charges that atoms in groups I, II, VI, and VII have in ionic compounds, by saying something like "noble gases are stable, therefore having 8 valence electrons must be stable for some reason. The octet rule is a useful way to quickly predict ionic charges and write Lewis structures, but it's not a magical law that atoms follow. What is really important is the total energy of the electron configuration. The electron configuration with the lowest energy is the most stable one.
The total energy of the electron configuration is determined by the energy of the electron orbitals and the effective nuclear charge "seen" by the electron.
The electronic structure of a fluorine atom is 1s2 2s2 2p5. There is a strong driving force for atoms to attain an octet (achieve an inert gas configuration) due to the extra stability associated with a filled shell of electrons. In order to complete its octet and achieve the neon inert gas configuration (1s2 2s2 2p6), fluorine must gain 1 electron and become the fluoride anion (F−). If it gains two electrons and becomes F−2, or loses 1 electron and becomes F+, it won't have an octet in its outer shell - it won't be anywhere near as stable as if it just gains one electron.
Application of Fluorine Ion
Fluorine is the most electronegative of all the elements and has a special affinity for electrons, which it readily accepts or offers to form electrical connections. It reacts with most organic compounds and is an oxidising acid that reacts rapidly with water and air to form dangerous acidic hydrogen fluoride (HF).
Fluoride ions carry a single negative charge, which makes them useful in a variety of chemical applications such as complex synthetic routes and other inorganic reactions. Fluorine is therefore a dangerous but essential element in chemistry, playing an important role in many chemical reactions and biological processes.