Carbon tetrachloride -Health Hazard and Toxicity
Carbon tetrachloride, also known as tetrachloromethane, has its molecule formula being CCl4. It appears as colorless liquid with the melting point of-23 ° C, boiling point of 76.8 ° C and the relative density of 1.5867. It can dissolve grease, paint, resin, rubber and many other substances, being commonly used organic solvent and extractant. It can also be used as dry cleaning agent. However, long-term exposure to carbon tetrachloride will irritate the skin, inhibit the central nervous system and cause damage to the liver and kidney. Therefore, the operator should pay special attention. Carbon tetrachloride is widely presented in the atmosphere, river water, sea water, seaweed and marine surface sediments. Its concentration in seawater is generally in ppb level. Carbon tetrachloride contained in red algae is estimated to be synthesized by the organism itself. The concentrations of carbon tetrachloride in the two hemispheres are very close, and are higher than the estimated amount based on production amount, which is related to the reaction of chlorine and methane in the atmosphere.

Toxicity Data
LD50 oral (rat) 2350 mg/kg
LD50 skin (rabbit) >20 g/kg
LC50 inhal (rat) 8000 ppm (4 h)
PEL (OSHA) 2 ppm (13 mg/m3)
TLV-TWA (ACGIH) 5 ppm (32.5 mg/m3)-skin
STEL (ACGIH) 10 ppm (65 mg/m3)
Major Hazards
Low to moderate acute toxicity; harmful to the liver, kidneys, and central nervous system.
Toxicity
The acute toxicity of carbon tetrachloride is low to moderate. Inhalation of carbon tetrachloride can produce symptoms such as dizziness, headache, fatigue, nausea, vomiting, stupor, and diarrhea. This substance is a depressant of the central nervous system, and inhalation of high concentrations causes damage to the liver, heart, and kidneys. Exposure to 1000 to 2000 ppm for 30 to 60 min can be fatal to humans. Ingestion of carbon tetrachloride leads to similar toxic effects, and swallowing as little as 4 mL can be lethal. Carbon tetrachloride irritates the skin, and prolonged contact may cause dryness and cracking. This substance is also slowly absorbed through the skin. Carbon tetrachloride liquid and vapor are also irritating to the eyes. The odor of carbon tetrachloride does not provide adequate warning of the presence of harmful concentrations. Carbon tetrachloride shows carcinogenic effects in animal studies and is listed by IARC in Group 2B ("possible human carcinogen"). It is not classified as a "select carcinogen" according to the criteria of the OSHA Laboratory Standard. Prolonged or repeated exposure to this substance may result in liver and kidney damage. There is some evidence from animal studies that carbon tetrachloride may be a developmental and reproductive toxin in both males and females.
Flammability
and Explosibility Carbon tetrachloride is noncombustible. Exposure to fire or high temperatures may lead to formation of phosgene, a highly toxic gas.
Reactivity and Incompatibility
Carbon tetrachloride may react explosively with reactive metals such as the alkali metals, aluminum, magnesium, and zinc. It can also react violently with boron and silicon hydrides, and upon heating with DMF.
Storage and Handling
Carbon tetrachloride should be handled in the laboratory using the "basic prudent practices" described in Chapter 5.C.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If carbon tetrachloride is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once.
Disposal
In the event of a spill, soak up carbon tetrachloride with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area. Disposal Excess carbon tetrachloride and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Reading extension
Properties of Carbon Tetrachloride
It was first discovered in 1839 by Henri Victor Regnault. He synthesized this compound by reacting chloroform with chlorine. These days it is mainly produced from methane.
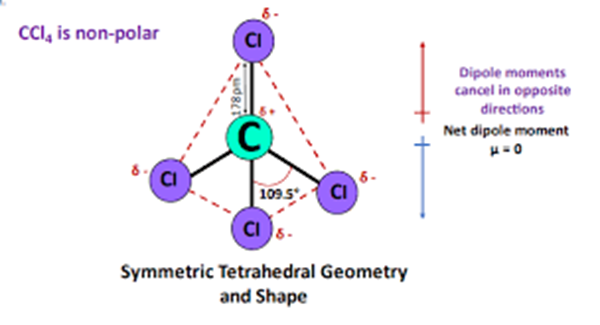
The positioning of four chlorine atoms in carbon tetrachloride is symmetric and hence it is a non-polar molecule.
Carbon Tetrachloride Uses
The non-polarity of Carbon Tetrachloride makes it an excellent solvent for non-polar substances.
It is also used as a source of chlorine in the synthesis of other chlorine-based organic compounds.
The absence of hydrogen atom in carbon tetrachloride makes it useful in NMR spectroscopy and its solvent properties make it a usable option for infrared spectroscopy.
Tetrachloromethane has been also used as a fire extinguisher in the past. It prevented fire by extinguishing the flames; its vaporization prevented the combustion reaction.
However, these uses of carbon tetrachloride were suppressed after the detection of toxic effects of this compound.
Effects of Carbon Tetrachloride
Carbon tetrachloride is a toxic material. Although it has a wide range of applications, its use has been highly suppressed.
Carbon tetrachloride hazards include severe damage to nervous system, kidneys, degenerate liver, it can even cause cancer.
It may lead to coma or even death in case of exposure to concentrated tetrachloromethane.Continued exposures to this gas can cause depression by affecting the nervous system.
Apart from its toxicity, it severely affects our environment. It is a greenhouse gas and has ozone-depleting properties and it is a significant cause of global warming and environmental damage.
The toxic effects of carbon tetrachloride have played the most important role in suppressing the use of this compound in different industries and thus preventing further damage to our environment. Its chemistry has been an important topic of research ever since its first synthesis.