Capmatinib: Pharmacodynamics, Pharmacokinetics and Adverse Events
General Description
Capmatinib is a potent and selective kinase inhibitor that targets MET exon 14 skipping mutations in non-small cell lung cancer. It inhibits MET phosphorylation and downstream effectors, impeding cell proliferation and migration while promoting apoptosis. Capmatinib has linear pharmacokinetics, with a dose-proportional increase in exposure and can be taken without regard to food. The most frequently reported adverse reactions include peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite. Serious adverse reactions were noted in 51% of patients, and one patient experienced a fatal adverse reaction. Adverse reactions led to permanent discontinuation of therapy in 16% of recipients, and dose interruptions were necessary for 54% of patients. Capmatinib has predictable pharmacokinetics, and its metabolism is well understood, enabling clinicians to manage potential drug interactions.

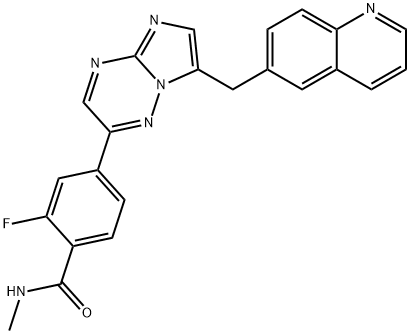
Figure 1. Capmatinib
Pharmacodynamics
The pharmacodynamic effect of capmatinib was measured at the end of the study by quantifying total MET and phospho-MET in tumor lysates using a multispot ELISA. LXFA 623 tumors showed markedly lower total and phospho-MET levels than the 2 MET-amplified models. MET inhibition was clearly detectable at 2 hours after the last dosing, with some degree of phospho-MET recovery in 2 out of 3 models at 12 hours after dosing.
In a third PDX model collection, a lung cancer model named LU5381 with MET exon 14 skipping mutation and moderate MET copy number gain (~5) was identified, thus dissociating MET exon 14 skipping from high-level MET amplification. When treating mice bearing LU5381 xenografts with capmatinib, we observed tumor regression. Notably, capmatinib was also active against a liver cancer xenograft model, in which the MET gene is amplified.1
Pharmacokinetics
Capmatinib is an orally administered drug with linear pharmacokinetics, exhibiting a dose-proportional increase in exposure (Cmax and AUC) between doses of 200-400 mg. Capmatinib is rapidly absorbed, with peak plasma concentrations achieved in 1-2 hours after a 400 mg dose. It is estimated that over 70% of the drug is absorbed after oral administration. The steady state is reached after three days of twice daily dosing, with an accumulation ratio of 1.5. Capmatinib can be taken without regard to food since it has similar exposure levels in both fasted and fed cancer patients. The plasma protein binding of capmatinib is 96%, and the mean apparent volume of distribution in the steady state is 164 L. Capmatinib is primarily metabolized by CYP3A4 and aldehyde oxidase, with 78% of total radioactivity recovered in feces (42% as unchanged drug) and 22% in urine. The effective elimination half-life of capmatinib is 6.5 hours, and the mean steady-state apparent clearance is 24 L/h. Coadministration of capmatinib with a strong CYP3A inhibitor increases capmatinib exposure, which may lead to more severe adverse reactions. Conversely, coadministration with a CYP3A inducer decreases capmatinib exposure, which may decrease its antitumor activity. A moderate CYP3A inducer may also decrease exposure to capmatinib if administered concomitantly. Overall, capmatinib has predictable pharmacokinetics, and its metabolism is well understood, enabling clinicians to manage potential drug interactions. 2
Adverse Events
Capmatinib monotherapy, administered at a dosage of 400 mg twice daily, demonstrated a favorable tolerability profile in non-small cell lung cancer (NSCLC) patients with MET exon 14 skipping mutations, as observed in the phase 2 GEOMETRY mono-1 study (NCT02414139). Among the 334 patients who received capmatinib, 31% were exposed to the medication for at least 6 months, and 16% for at least 1 year. The most frequently reported adverse reactions (with an incidence of ≥20%) of any grade included peripheral edema (52%), nausea (44%), fatigue (32%), vomiting (28%), dyspnea (24%), and decreased appetite (21%). Grade 3 or 4 adverse reactions with an incidence of >5% consisted of peripheral edema (9%), fatigue (8%), and dyspnea (7%). Serious adverse reactions were noted in 51% of patients, with dyspnea (7%), pneumonia (4.8%), pleural effusion (3.6%), general physical health deterioration (3%), vomiting (2.4%), and nausea (2.1%) being the most commonly reported. One patient experienced a fatal adverse reaction (pneumonitis) while receiving capmatinib. Adverse reactions led to permanent discontinuation of therapy in 16% of recipients, with peripheral edema (1.8%), pneumonitis (1.8%), and fatigue (1.5%) being the most common reasons. Dose interruptions were necessary for 54% of patients, primarily due to peripheral edema, increased blood creatinine, nausea, and vomiting. Additionally, 23% of recipients required dose reductions due to adverse reactions, with peripheral edema, increased alanine transferase, increased blood creatinine, and nausea being the most prevalent reasons. Other clinically relevant adverse reactions reported in less than 10% of patients included pruritus, interstitial lung disease/pneumonitis, cellulitis, acute kidney injury (including renal failure), urticaria, and acute pancreatitis. 2
References:
[1] SABRINA BALTSCHUKAT. Capmatinib (INC280) Is Active Against Models of Non-Small Cell Lung Cancer and Other Cancer Types with Defined Mechanisms of MET Activation.[J]. ACS Central Science, 2019. DOI:10.1158/1078-0432.CCR-18-2814.[2] DHILLON S. Capmatinib: First Approval.[J]. ACS Chemical Neuroscience, 2020. DOI:10.1007/s40265-020-01347-3.
You may like
Related articles And Qustion
Lastest Price from Capmatinib manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 1029712-80-8
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 10kg/month
US $0.00-0.00/mg2025-04-18
- CAS:
- 1029712-80-8
- Min. Order:
- 10mg
- Purity:
- 98
- Supply Ability:
- 100000