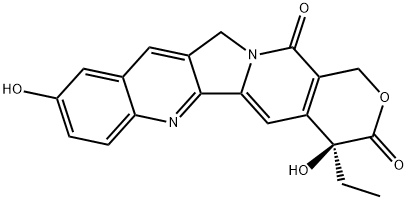
Camptothecin vs. 10-Hydroxycamptothecin: Uses and Synthesis
Camptothecin (CPT) is a pentacyclic natural alkaloid extracted from Camptotheca acuminata. CPT is a unique class of complex quinoline cytotoxins that inhibit the DNA enzyme topoisomerase I (topo I). 10-Hydroxycamptothecin is a derivative of camptothecin, in which the hydrogen bond at the 10th position of the benzene ring is replaced by a hydroxyl group (-OH). 10-Hydroxycamptothecin is a pyranoindolequinoline compound that can be used as an industrial raw material for the production of anticancer drugs topotecan and irinotecan.
Synthesis
Camptothecin and 10-Hydroxycamptothecin were mainly completed by the following three steps:
(1) Construction of Quinoline Precursors 6 by Pyrrolidine-Catalyzed Michael Addition-Aldol Condensation Cascade Reaction
In the presence of catalytic amounts of pyrrolidine (10 mol %) and benzoic acid (10 mol %), the reaction of R,-unsaturated aldehyde 8 with 2-aminobenzaldehyde 7 can be carried out smoothly in dichloromethane at room temperature to obtain two inseparable products. After filtering to remove the silica gel, the resulting solution was directly treated with freshly prepared MnO2 to obtain quinoline 6 with an overall yield (75% for 6a and 72% for 6b).
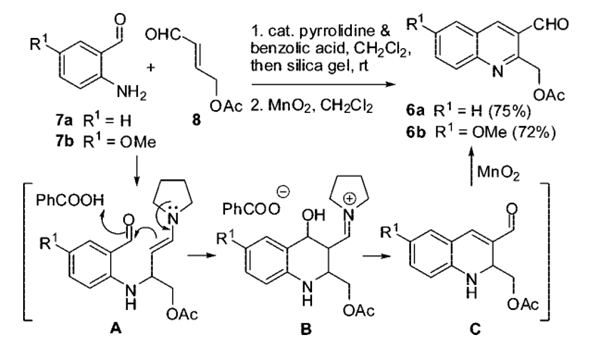
(2) Syntheses of ABC-Ring Intermediates 12
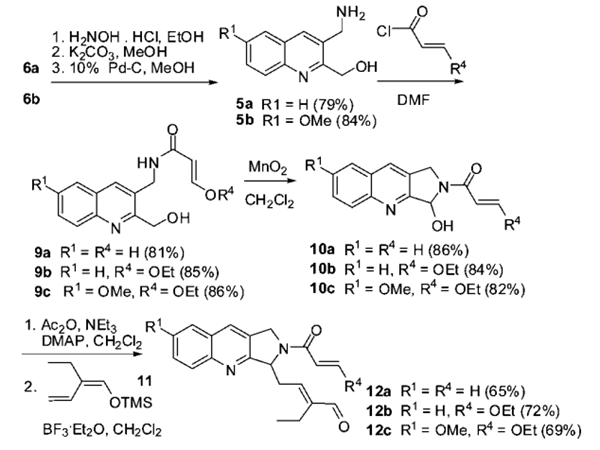
The quinolinyl binders 6 were then converted to the corresponding amine precursors 5 in a three-step sequential process (without purification of the intermediates). Benzaldehyde 6 was oximated with NH2OH, the acetate was removed with K2CO3 in methanol, and the oxime was then hydrogenated with 10% Pd-C in methanol at room temperature (1 atm) to afford the corresponding benzylamines 5 in good yields (79% for 5a and 84% for 5b). Amines 5 were acylated in parallel with acryloyl chloride or 3-ethoxyacryloyl chloride in DMF to afford the corresponding acrylamides 9 (81% for 9a, 85% for 9b, and 86% for 9c). A mild MnO2-based oxidation of quinolin-2-ylmethanol 9 in dichloromethane afforded the aminal derivative 10 (86% for 10a, 84% for 10b, and 82% for 10c). The aminal 10 was acetylated in the presence of BF3·Et2O at -78 °C and then treated with the enol silyl ether 11 to afford the substrate 12 (65% for 12a, 72% for 12b, and 69% for 12c) for the following oxa Diels-Alder reactions.
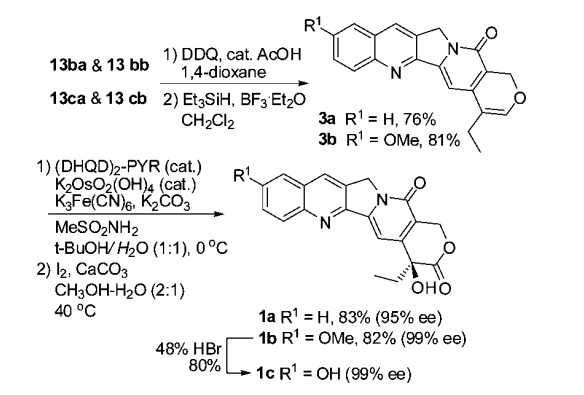
Introduction of an electron-donating ethoxy group into the unsaturated amide substrate 12 followed by thermal cycloaddition gave two separable diastereoisomers (13ba and 13bb, 3:1) in 83% yield.
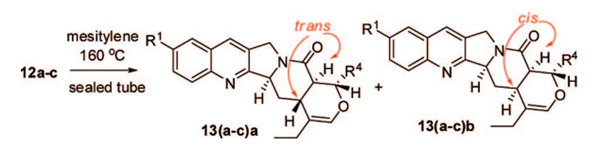
(3) Completion of Total Syntheses of Camptothecin and 10-Hydroxyoxycamptothecin
Once pentacyclic precursor 13 was available, it was used to synthesize CPT and 10-OH CPT. A mixture of 13ba and 13bb was treated with DDQ and catalytic CH3COOH in 1,4-dioxane 15, followed by reductive removal of the ethoxy group 16 to afford CPT precursor 3a in 76% yield. Thus, another mixture 13ca and 13cb gave 3b in 81% yield. Further oxidative transformation (Sharpless asymmetric dihydroxylation followed by I2/CaCO3-based hemiacetal oxidation) 8a successfully converted cyclic enol ethers 3a and 3b to camptothecin (1a) and 10-methoxycamptothecin (1b), respectively. In addition to camptothecin (83% yield, 95% ee), 8a also gave 10-methoxycamptothecin ( 1b ) in 82% yield and 99% ee.
References:
[1] GUAN-SAI LIU. Expeditious Total Syntheses of Camptothecin and 10-Hydroxycamptothecin[J]. Organic Letters, 2008, 10 23: 5321-5499. DOI:10.1021/ol802250y.You may like
See also
Lastest Price from 10-Hydroxycamptothecin manufacturers

US $0.00/kg2025-04-21
- CAS:
- 19685-09-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg
US $0.00/mg2023-02-24
- CAS:
- 19685-09-7
- Min. Order:
- 5mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g