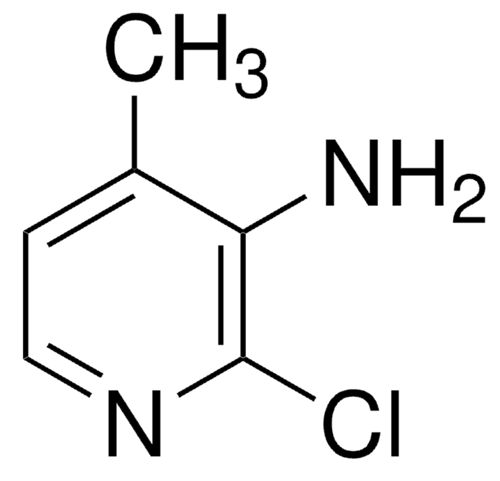
Brominated Polystyrene: Applications as Fireproofing Agents, Preparation and Degradation Mechanism
General Description
Brominated polystyrene serves as an effective flame retardant in thermoplastic polymer compositions, particularly in glass-filled polyesters and nylons. Its unique properties, including high thermal stability, low chlorine content, and absence of impurities, contribute to superior flame resistance. The preparation process involves careful control of reactant concentration, catalyst selection, brominating agent, and temperature to achieve enhanced color characteristics. Analytical techniques reveal that the presence of bromine atoms influences the degradation mechanism, leading to the formation of brominated aromatic compounds during pyrolysis. Understanding these processes is crucial for optimizing the performance and applications of brominated polystyrenes.

Figure 1. Brominated polystyrene
Applications as Fireproofing Agents
Brominated polystyrene, when used as fireproofing agents, offers a range of applications due to its unique properties. These novel brominated styrenic polymers, with low ionic bromine content and specific characteristics, serve as effective flame retardant additives in various thermoplastic polymer compositions. One key application lies in the field of thermoplastics, particularly in glass-filled polyesters and glass-filled nylons. When blended with a thermoplastic polymer, brominated polystyrene acts as a flame retardant, imparting superior performance qualities to the resulting polymer composition. The exceptional flame retardancy of brominated polystyrenic resins is attributed to their specific characteristics. These include a high TGA temperature for 1 weight loss (>340°C), low chlorine content (if present, less than about 700 ppm Cl), and absence of impurities such as ethylene dichloride, bromodichloroethane, dibromochloroethane, dibromodichloroethane, and tribromochloroethane. Compared to traditional brominated polystyrenes, the brominated styrenic polymers offer enhanced thermal stability, reduced metal corrosiveness, and improved overall performance. The blends produced with these novel flame retardants exhibit superior fire resistance, making them suitable for applications where fire safety is critical. In summary, brominated polystyrene finds extensive use as a fireproofing agent in thermoplastics, providing enhanced flame retardancy and ensuring the safety of various products and materials. 1
Preparation
The process for preparing brominated polystyrene with enhanced color characteristics involves several key steps. Firstly, a solution of a polystyrene reactant containing around 5-20% of the reactant in a halogenated hydrocarbon solvent is prepared. Subsequently, a Lewis acid bromination catalyst is added to the solution, followed by the addition of approximately 1-3.3 mol of a brominating agent per mol of polystyrene repeating units. The reaction between the polystyrene reactant and the brominating agent occurs within a temperature range of about -20° to 50°. The improvement in this process lies in controlling the color properties of the resulting brominated polystyrene. This is achieved by selecting a polystyrene reactant with a molecular weight (Mw) ranging from 500 to 1,500,000. Furthermore, the selection of an appropriate amount of catalyst is crucial to ensure monobromination of the polystyrene without unwanted alkylation by the solvent. The choice of brominating agent, such as bromine chloride or bromine, also impacts the color characteristics. Operating at the lowest feasible temperature within the specified range, based on the selected brominating agent and catalyst, is essential. Lastly, isolating the brominated polystyrene while considering factors like reaction time, temperature, catalyst, brominating agent, and isolation method further contributes to improving the color properties of the final product. By meticulously controlling these variables, the process results in brominated polystyrene with superior color characteristics. 2
Degradation Mechanism
The degradation mechanism of brominated polystyrenes involves several key processes that were elucidated through analytical techniques such as pyrolysis-gas chromatography/mass spectrometry and thermogravimetric analysis. When subjected to thermal conditions, brominated polystyrenes undergo pyrolysis, resulting in the breakdown of chemical bonds and the release of various pyrolysis products. Analysis of these products provides insights into the degradation pathway of the polymers. Specifically, the presence of bromine atoms in the polymer chain influences the thermal behavior and degradation mechanism. The bromine substituents can lead to the formation of brominated aromatic compounds during pyrolysis. Additionally, the nature and composition of the pyrolysis products offer valuable information about the stability and reactivity of brominated polystyrenes under different temperature conditions. Overall, this study of the thermal behavior of brominated polystyrenes highlights the importance of understanding the degradation mechanism to optimize their performance and applications in various fields. 3
Reference
1. Dadgar BB, Galhoff DE. Brominated polystyrenic resins as fireproofing agents. 2001; Patent Number: US6232408.
2. Dever JL, Gill JC. Process for preparation of brominated polystyrene having improved color characteristics. 1998; Patent Number: US5723549.
3. Bertini F, Kiji J, Audisio G. Thermal behavior and degradation mechanism of brominated polystyrenes. Journal of Analytical and Applied Pyrolysis. 1995; 33: 213-230.
Related articles And Qustion
Lastest Price from Brominated polystyrene manufacturers

US $0.00-0.00/KG2025-05-13
- CAS:
- 88497-56-7
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $10.00/kg2025-04-15
- CAS:
- 88497-56-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton