Brigatinib: Development History and Preclinical Studies
General Description
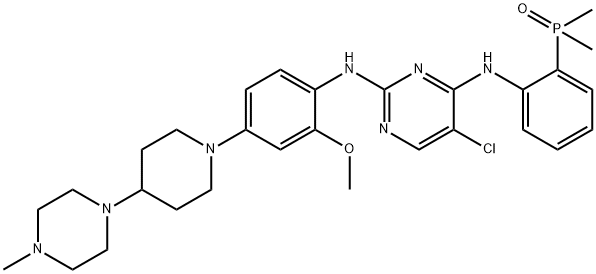
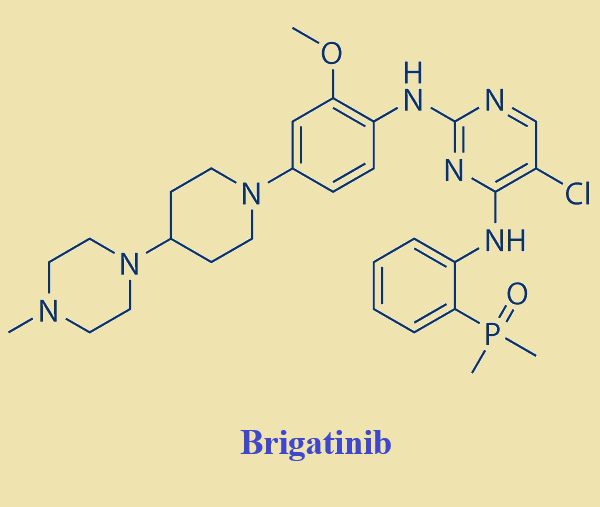
Brigatinib is a vital treatment for non-small cell lung cancer patients with EGFR and ALK genetic mutations. Approved by the FDA in 2017, its development involved significant milestones, including successful clinical trials and FDA acceptance. The drug's chemical features enable it to effectively inhibit ALK, with an MIC value of 0.6 nmol/L. Brigatinib's unique structure allows it to bind to the ATP attachment site of ALK, demonstrating potent activity against mutations, including those resistant to other ALK inhibitors. These features position Brigatinib as a robust and selective ALK inhibitor, offering hope for non-small cell lung cancer patients facing resistance to traditional treatments.

Figure 1. Brigatinib
Development history1
Lung cancer is one of the deadliest forms of cancer. It is associated with high death rate accounting alone for 1/5th of total cancer mortality worldwide. It has a very poor survival rate and prognosis, as more than half of the diagnosed patients die within one year of diagnosis. Lung cancer is subdivided into two types; Small cell lung carcinoma (SLCC) and Non-small cell lung cancer (NSCLC). NSCLC is the most common kind of lung cancer which is associated with an increase in production of epithelial lung cells and accounts approximately for 85–90% of all lung cancer cases.
Mutations in EGFR and ALK genetic arrangements are the leading cause of death in lung cancer patients. Currently it is the drug of choice for providing treatment to patients that are resistant to other ALK inhibitors.
Update on Brigatinib from 2015 to 2017:
1. On 17th April 2015, ARIAD pharmaceuticals showed updated information on the ongoing clinical trials of brigatinib in patients that were suffering from NSCLC caused due to ALK genetic rearrangements.
2. In April 2016, phase ½ trial was completed and company updated the latest information about this trial.
3. After completion of clinical trial studies, the ARIAD pharmaceuticals filed a New Drug Application for brigatinib in U.S. and FDA on 17th June 2016.
4. On 30th August 2016, completion of submission of New Drug Application in U.S. and Food and Drug Administration.
5. On 31st October 2016, the manufacturer announced that FDA accepted the new drug application file.
6. On April 28th 2017 TAKEDA announced that FDA has approved the brigatinib for market usage.
Preclinical studies2
A kinase screen was performed by Victor M. Riverato et.al. to evaluate the selectivity profile of brigatinib. The cellular and in vivo activities of ALK TKIs were compared using engineered and cancer-derived cell lines. The brigatinib–ALK co-structure was determined.
The result shows that brigatinib potently inhibits ALK and ROS1, with a high degree of selectivity over more than 250 kinases. Across a panel of ALK+ cell lines, brigatinib inhibited native ALK (IC50, 10 nmol/L) with 12-fold greater potency than crizotinib. Superior efficacy of brigatinib was also observed in mice with ALK+ tumors implanted subcutaneously or intracranially. Brigatinib maintained substantial activity against all 17 secondary ALK mutants tested in cellular assays and exhibited a superior inhibitory profile compared with crizotinib, ceritinib, and alectinib at clinically achievable concentrations. Brigatinib was the only TKI to maintain substantial activity against the most recalcitrant ALK resistance mutation, G1202R. The unique, potent, and pan-ALK mutant activity of brigatinib could be rationalized by structural analyses.
In summarty, brigatinib is a highly potent and selective ALK inhibitor. These findings provide the molecular basis for the promising activity being observed in ALK+, crizotinib-resistant patients with NSCLC being treated with brigatinib in clinical trials.
References:
[1] SILKY BEDI . A comprehensive review on Brigatinib – A wonder drug for targeted cancer therapy in non-small cell lung cancer[J]. Saudi Pharmaceutical Journal, 2018, 26 6: 755-908. DOI:10.1016/j.jsps.2018.04.010.[2] SEN ZHANG. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models[J]. Clinical Cancer Research, 2016, 22 1: 5527-5538. DOI:10.1158/1078-0432.CCR-16-0569.
You may like
Related articles And Qustion
Lastest Price from Brigatinib manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 1197953-54-0
- Min. Order:
- 1kg
- Purity:
- 99.0%
- Supply Ability:
- 10000
US $0.00-0.00/g2025-04-18
- CAS:
- 1197953-54-0
- Min. Order:
- 50g
- Purity:
- 99%
- Supply Ability:
- 8kgs