Botulinum toxin and Botulinum toxin A
Introduction
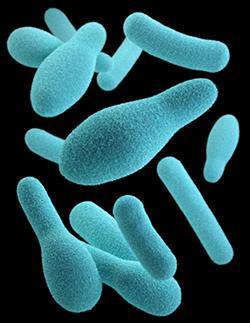
Botulinum toxin (abbreviated either as BTX or BoNT) is produced by Clostridium botulinum, a gram-positive anaerobic bacterium. The clinical syndrome of botulism can occur following ingestion of contaminated food, from colonization of the infant gastrointestinal tract, or a wound infection.
BoNT is broken into 7 neurotoxins (labeled as types A, B, C [C1, C2], D, E, F, and G), antigenically and serologically distinct but structurally similar. Human botulism is caused mainly by types A, B, E, and (rarely) F. Types C and D cause toxicity only in animals.
Use method
Botulinum toxin is injected into affected muscles or glands using a 30-gauge 1-inch needle. Doses are tailored according to the mode of use and individual patients, and the dose depends on the muscle mass being injected: The larger the muscle mass, the higher the dose required. However, lower doses may be required in patients with preexisting weakness and females.
Toxin injections are given through hollow teflon-coated needles directly into affected/overactive muscles. In localized muscle overactivity, especially in delicate places such as strabismus, the injections are usually guided by electromyography.
Botulinum toxin A
Two preparations of botulinum toxin A exist Dysport® and Botox®. Unfortunately, there has been much confusion over the doses and units of potency of the two preparations[1]. Although doses are quoted in mouse units (which is the amount of toxin that kills 50% of a group of 18-20 g female Swiss-Webster mice), implying some standardization, Botox® seems more potent. Recently, it has been shown that a unit of Botox® is three times as potent as a unit of Dysport®.
Botox® is a sterile lyophilized form of botulinum toxin type A. It is produced from a culture of the Hall strain of C. botulinum and purified by a series of acid precipitations to a crystalline complex containing the toxin and other proteins. The FDA approved Botox® in December 1989 as an orphan drug for treating strabismus, hemifacial spasms, and blepharospasm. The specific activity of Botox® is approximately 20 Units/ nanogram of the neurotoxin protein complex. Each vial of Botox® contains 100 Units (U) of Clostridium botulinum type A neurotoxin complex, 0.5 milligrams of Albumin (Human), and 0.9 milligrams of sodium chloride in a sterile, vacuum-dried form without a preservative.
Dysport®, another formulation of botulinum toxin type A available in Europe and a few other countries, is prepared using column-based purification techniques and distributed in 500-unit vials that can be stored at room temperature. Botox® and Dysport® are both botulinum toxin type A preparations but are quite distinct from one another. Differences in these toxins may relate to differences in the strain of bacterium, preparation, diffusion, and potency testing.
References
[1] P K Nigam, Anjana Nigam. “Botulinum toxin.” Indian Journal of Dermatology 55 1 (2010): 8–14.
You may like
Related articles And Qustion
See also
Lastest Price from Botulinum toxin manufacturers

US $0.00/box2025-09-24
- CAS:
- 93384-43-1
- Min. Order:
- 1box
- Purity:
- 0.99
- Supply Ability:
- 10tons

US $20.00/box2025-08-20
- CAS:
- 93384-43-1
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock