Biological Activity of Poziotinib (HM781-36B)
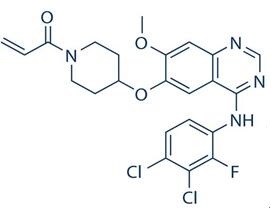
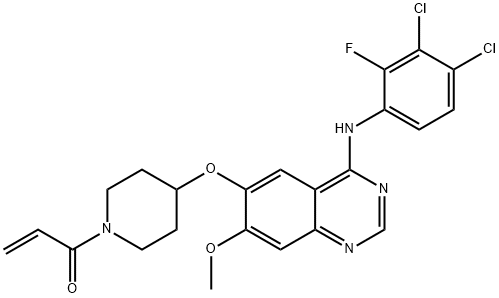
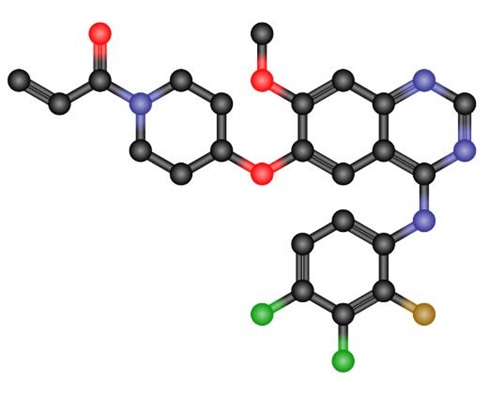
Figure 1. Structural formula of poziotinib (HM781-36B)
Poziotinib [1. {4. [4. (3,4. Dichloro.2.monofluoroaniline) -7-methoxyquinazoline-6. Oxygen]. 1. Piperidinyl} propan-2-ene-1one (1)] is a novel oral cancer cell inhibitor developed by Seoul Medical University in South Korea in 2008. It is used for the treatment of breast and gastric cancer, and has targeted Small molecule inhibitor of tyrosine kinase. It is currently in Phase II / III clinical research and can be used alone or in combination for the treatment of gastric, non-small cell lung cancer and breast cancer. Poziotinib is resistant to erlotinib and gefitinib EGFR L858R / T790M Double mutant cells have a strong inhibitory effect. The combined use of Poziotinib (HM781-36B) and 5-fluorouracil, platinum compound, paclitaxel or gemcitabine shows a good synergistic inhibitory effect on the overexpression of human epidermis No. 2 (HER2). A new third-generation anti-tumor cell inhibitor following the kinase inhibitor afatinib.
Poziotinib is an innovative oral broad-spectrum HER inhibitor. It can irreversibly block signaling of all HER family tyrosine kinase receptors.
For HER receptors carrying the exon 20 insertion mutation, preclinical tests have shown that poziotinib inhibits them dozens of times than existing tyrosine kinase inhibitors.
Poziotinib is currently undergoing multiple clinical studies for breast cancer, lung cancer, stomach cancer, head and neck cancer and other indications. Spectrum Pharmaceuticals has announced the interim results of a Phase 2 clinical trial of poziotinib in patients with non-small cell lung cancer (NSCLC) carrying EXON 20 insertion/mutation, indicating that the patient's objective response rate is 73%. The results of the experiment were announced at the 18th World Congress of Lung Cancer, International Association for the Study of Lung Cancer, IASLC.
The University of Texas MD Anderson Cancer Center conducted an open-label, single-arm phase II trial in which 25 non-small cell lung cancer patients with an EGFR exon 20 insertion mutation were treated with poziotinib.
In the second phase of the clinical trial, the tumors of 11 (73%) patients with non-small cell lung cancer all shrank to varying degrees, and the tumors of 8 patients shrank by at least 30%. It was concluded that the response rate of poziotinib was 73%. In addition, researchers also found that central nervous system metastases in one patient also responded to the drug.
Six of the 11 patients had their doses reduced due to side effects. The toxic side effects of the drug mainly included rash, diarrhea, paronychia and mucositis. These side effects are similar to those of previous trials of poziotinib and other tyrosine kinase inhibitors.
In in vitro research: Poziotinib specifically inhibits the growth of HER2 amplified gastric cancer cells, and inhibits EGFR phosphorylation and key components of downstream signal cascade amplification, such as STAT3, AKT and ERK. Poziotinib also induces apoptosis and G1 cell cycle arrest by activating the mitochondrial pathway in HER2 amplified gastric cancer cells. In addition, Poziotinib plays a synergistic role with chemotherapeutic agents in HER2-induced and HER2-non-expanded gastric cancer cells.
In in vivo research: In nude mice bearing N87 human gastric cancer xenografts, Poziotinib (0.5 mg/kg p.o.) alone significantly inhibited tumor growth, and Poziotinib combined with 5-FU showed more effective tumor suppression. In addition, in various EGFR and HER-2 dependent tumor xenograft models, including erlotinib-sensitive HCC827 NSCLC cells, erlotinib-resistant NCI-H1975 NSCLC cells, HER-2 overexpressing Calu-3 NSCLC cells, NCI-N87 Among gastric cancer cells, SK-Ov3 ovarian cancer cells and EGFR-overexpressing A431 epidermal-like cancer cells, HM781-36B showed excellent antitumor activity.
Application Outlook:
Poziotinib is suitable for the treatment of patients with advanced NSCLC with exon 20 mutations in EGFR. Multiple patients have proven effective. Poziotinib is also valuable for patients with advanced NSCLC who are resistant to first-generation EGFR TKI and T790M / MET negative. There are no clinical data on the treatment of patients with brain metastases by Poziotinib. One patient with advanced NSCLC with exon 20 mutation in EGFR had meningeal metastases. This patient has been taking Poziotinib for effective control for 4 months.
References
[1] International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer; Yokohama, Japan: October 15-18, 2017.
[2] Mingsha S, Shuchao M, Lei Y, et al. Synthesis of d3-Poziotinib Hydrochloride and Stability of Liver Microsomes in vitro[J]. Journal of Yantai University(Natural Science and Engineering Edition), 2019, 32(3): 220-225.
[3] WANG Jian ya, MA Wei ying, CAI Zhi qiang. Process Improvement on the Synthesis of Poziotinib[J]. Chinese Journal of Synthetic Chemistry, 2015, 23,7: 664-667.
[4] http://www.chemspider.com/Chemical-Structure.30687714.html?rid=fb5ca1ab-a9f0-4ba7-9fc9-4dd905a3687b
[5] https://pubchem.ncbi.nlm.nih.gov/compound/25127713
You may like
Related articles And Qustion
See also
Lastest Price from Poziotinib (HM781-36B) manufacturers

US $0.00/g2025-04-21
- CAS:
- 1092364-38-9
- Min. Order:
- 10g
- Purity:
- 98%min
- Supply Ability:
- 1000g

US $0.00/kg2025-04-20
- CAS:
- 1092364-38-9
- Min. Order:
- 1kg
- Purity:
- 99% HPLC
- Supply Ability:
- 1000kg