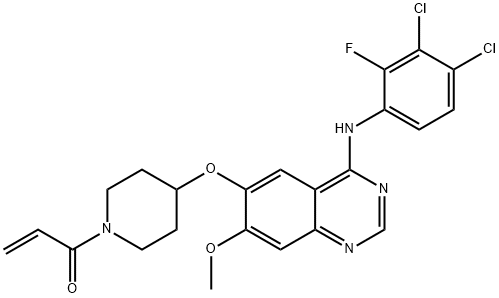
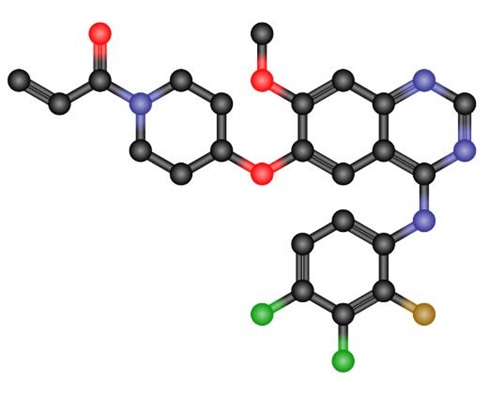
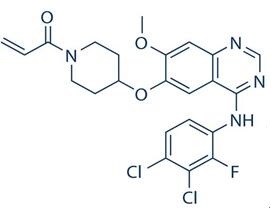
Poziotinib: mechanism of action, metabolism, clinical applications, side effects
General Description
Poziotinib is a targeted tyrosine kinase inhibitor used in the treatment of non-small cell lung cancer (NSCLC), breast cancer, and gastric cancer. It specifically targets HER2 amplification and suppresses EGFR signaling pathways, leading to the inhibition of cancer cell growth. Poziotinib demonstrates excellent anti-tumor activity in various tumor models, both HER2-dependent and non-amplified, and shows synergy with chemotherapy agents. Common adverse reactions of Poziotinib, similar to afatinib, include grade 3 side effects such as diarrhea, stomatitis, mucositis, rash, decreased appetite, pruritus, paronychia, hypokalemia, and acneiform dermatitis. Overall, Poziotinib is a promising third-generation anti-tumor cell inhibitor, particularly effective against HER2 amplification and resistant mutations, and it exhibits potent anti-tumor activity in various tumor models.

Figure 1. Capsules of poziotinib
Mechanism of action
Poziotinib inhibits the growth of HER2-amplified gastric cancer cells and suppresses the phosphorylation of EGFR and key downstream signaling components, such as STAT3, AKT, and ERK. It also induces apoptosis and G1 cell cycle arrest through the activation of a mitochondrial pathway in HER2-amplified gastric cancer cells. Moreover, Poziotinib demonstrates synergy with chemotherapy agents in both HER2-induced and non-amplified gastric cancer cells. In xenograft mouse models with N87 human gastric cancer, Poziotinib (0.5 mg/kg p.o.) alone significantly inhibits tumor growth, while its combination with 5-fluorouracil shows even more effective tumor suppression. Additionally, Poziotinib exhibits excellent anti-tumor activity in various EGFR and HER2-dependent tumor xenograft models, including erlotinib-sensitive HCC827 NSCLC cells, erlotinib-resistant NCI-H1975 NSCLC cells, HER-2 overexpressed Calu-3 NSCLC cells, NCI-N87 gastric cancer cells, SK-Ov3 ovarian cancer cells, and EGFR overexpressed A431 epidermoid carcinoma cells. In summary, Poziotinib inhibits cancer cell growth by specifically targeting HER2 amplification and suppressing EGFR signaling pathways. It also synergizes with chemotherapy agents and demonstrates potent anti-tumor activity in various tumor models. 1
Metabolism
Poziotinib is a medication that is metabolized and eliminated from the body following first-order elimination kinetics. This means that the rate of elimination is proportional to the concentration of the drug in the body. The half-life of poziotinib is approximately 6.6 hours, which means that it takes about 6.6 hours for the concentration of the drug in the body to decrease by half. The average distribution volume of poziotinib is 164 liters, and its average apparent clearance rate is 34.5 liters per hour. After oral administration, poziotinib reaches its peak blood concentration approximately 1.75 hours later. Interestingly, food intake does not appear to affect the blood concentration of poziotinib. However, patient weight can influence the absorption and blood concentration of the drug. 2
Clinical Applications
Poziotinib exhibits excellent anti-tumor activity in various EGFR and HER-2 dependent tumor xenograft models, including erlotinib-sensitive HCC827 NSCLC cells, erlotinib-resistant NCI-H1975 NSCLC cells, HER-2 overexpressing Calu-3 NSCLC cells, NCI-N87 gastric cancer cells, SK-Ov3 ovarian cancer cells, and EGFR overexpressing A431 epidermoid carcinoma cells. Poziotinib is a novel oral cancer cell inhibitor used in the treatment of NSCLC, breast cancer, and gastric cancer. It acts as a targeted tyrosine kinase inhibitor with strong inhibitory effects on EGFR L858R/T790M double-mutant cells resistant to gefitinib and erlotinib. Poziotinib, when combined with 5-fluorouracil, platinum compounds, paclitaxel, or gemcitabine, exhibits excellent synergistic inhibitory effects on overexpressed human epidermal growth factor receptor 2. It represents a new third-generation anti-tumor cell inhibitor following the second-generation tyrosine kinase inhibitor, afatinib. 2
Side effects
Poziotinib is a medication that has been shown to have common adverse reactions that are similar to those of afatinib. The most frequently reported grade 3 adverse reactions include diarrhea, stomatitis, mucositis, rash, decreased appetite, pruritus, paronychia, hypokalemia, and acneiform dermatitis. These side effects can be uncomfortable for patients and may require medical attention. However, it is important to note that grade 4 and 5 adverse reactions have not been reported in clinical studies. 3
Reference
1. Ren S, Wang J, Ying J, et al. Consensus for
HER2 alterations testing in non-small-cell lung cancer. ESMO Open, 2022,
7(1):100395.
2. Remon J, Hendriks LEL, Cardona AF, Besse B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev, 2020, 90:102105.
3. POZIOTINIB: PATIENT INFORMATION-How to Manage Side Effects. Poziotinib-lung-cancer-study, 2018. (http://www.poziotinib-lung-cancer-study.com/images/POZI-1710-01900-PatientBrochure_r8.pdf)
Related articles And Qustion
See also
Lastest Price from Poziotinib (HM781-36B) manufacturers

US $0.00/g2025-04-21
- CAS:
- 1092364-38-9
- Min. Order:
- 10g
- Purity:
- 98%min
- Supply Ability:
- 1000g

US $0.00/kg2025-04-20
- CAS:
- 1092364-38-9
- Min. Order:
- 1kg
- Purity:
- 99% HPLC
- Supply Ability:
- 1000kg