Biological actions and interactions of tetrachloroethylene
General description
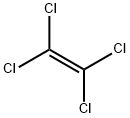
Tetrachloroethylene is a colorless, volatile, nonflammable, liquid, chlorinated hydrocarbon with an ether-like odor that may emit toxic fumes of phosgene when exposed to sunlight or flames with a characteristic odor. Its lipophilic character, stability, nonflammability and relatively high boiling point of 121°C (vapor pressure at 20°C: 1.8 kP) makes it an ideal solvent. Per is extensively used world-wide as a dry-cleaning solvent for textiles, for processing and finishing, for metal cleaning and extraction, as a heat-exchange fluid or as solvent in the manufacture of rubber solutions, for fats, oils, silicones and sulfur. Additionally, Per serves as a chemical intermediate in the synthesis of other halogenated hydrocarbons, such as various fluorocarbons or hexachloroethane[1].
Application
Tetrachloroethylene has been one of the most widely used chlorinated solvents in the United States. Tetrachloroethylene is mainly used as a cleaning solvent in dry cleaning and textile processing and in the manufacture of fluorocarbons. Tetrachloroethylene is a manufactured chemical that is widely used for dry cleaning of fabrics and for metal-degreasing. It is also used to make other chemicals and is used in some consumer products. Tetrachloroethylene is a natural product found in Gossypium hirsutum with data available.
At present, the infrared spectrophotometry with carbon tetrachloride as the extractant is widely used in China's petroleum monitoring methods. However, according to the Montreal Protocol on ozone depleting substances signed by China in 1987, carbon tetrachloride is determined as the reagent banned globally, and China must stop using it all by 2014. Therefore, finding alternatives to carbon tetrachloride has become a top priority. In recent years, we have consulted a large number of relevant literature on the determination of petroleum and the determination of oil fume in catering industry, and believe that the following substances may be used as alternatives to carbon tetrachloride: hexachloro tetrafluorobutane (s-316), h997, carbon disulfide and tetrachloroethylene[2].
Synthesis
With the continuous development and popularization of green refrigerants in the future, the consumption of tetrachloroethylene in the field of chemical intermediates will continue to increase. At present, the production methods of tetrachloroethylene mainly include acetylene method, ethylene direct chlorination method, thermal chlorination method, oxychlorination method, among which oxychlorination is the most advanced. Oxychlorination method is widely used abroad, while acetylene method is mainly used in China, which has serious energy consumption and pollution. Due to the difference of technical routes, the quality of domestic tetrachloroethylene can not meet the requirements of the dry cleaning industry, so it is only limited to organic synthesis and metal degreasing, while tetrachloroethylene, which occupies the mainstream market of dry cleaning agents, needs to be imported [6]. With the development of chlor alkali industry and chlorine related products, the capacity of by-product hydrogen chloride in China has been seriously surplus[2,3].
1.There are great industrial value and good environmental effects in converting by-products from methane chloride to the chloride containing more carbons. In this study, tetrachlorethylene generates by dehydrochlorination of chloroform couple, and it is the key to finding an appropriate catalyst. The catalysts were synthesized by dipping,while activated carbon and 13X zeolite were chosen as the carriers respectively, and metal chloride was chosen as the active component. Under the condition of reaction temperature 400℃, weight hourly space velocity 1h-1 , the catalyst FeCl3-AC had best catalytic activity. The conversion of chloroform is 94.1%, the selectivity of tetrachloroethylene is 30.9 %.
2.The reaction system of oxychlorination of 1,2-dichloroethane, oxygen and hydrogen chloride to tetrachloroethylene was thermodynamically analyzed. The effects of reaction temperature, reaction space velocity, oxygen and the amount ratio of hydrogen chloride to 1,2-dichloroethane on the reaction results were investigated through experiments under the direct action of Deacon reaction catalyst. Thermodynamic analysis shows that the total reaction to produce tetrachloroethylene is a spontaneous and complete exothermic reaction, and appropriately reducing the temperature is conducive to the reaction. The experimental results show that the optimum process conditions are as follows: reaction temperature 410 ℃, space velocity 0.54h-1, and the amount ratio of hydrogen chloride, oxygen and 1,2-dichloroethane is 2.76 ∶ 1.97 ∶ 1. Under these conditions, the conversion of 1,2-dichloroethane was 99.20% and the selectivity of tetrachloroethylene was 86.23%.
Figure 1 the synthesis route of C2Cl4(tetrachloroethylene)
Safety
Tetrachloroethylene is reasonably anticipated to be a human carcinogen and may be linked to an increased risk of developing skin, colon, lung, esophageal, and urogenital tract cancer as well as lymphosarcoma and leukemia. (NCI05). Exposure to this substance irritates the upper respiratory tract and eyes and causes neurological effects as well as kidney and liver damage.
Within the dry cleaning industry, the overall arithmetic mean (AM) for personal tetrachloroethylene exposures was 59 ppm (range:0–4636,n=1395). Machine operators who transferred wet garments to a dryer had the highest levels (AM=150 ppm [range: 0–1000, n=441]) of the jobs in this industry. The AM for personal measurements associated with degreasing was 95 ppm (range: 0–1800, n=206). In addition, we identified several other sources of substantial tetrachloroethylene exposure, including cleaning mining equipment, testing coal, cleaning animal coats in taxidermy, and cleaning and duplicating film. Exposure assessment in population-based, case-control studies is a complex process requiring substantial resources. Researchers conducting these types of studies will be able to use results of the measurements to quantify tetrachloroethylene exposure levels for various jobs.
References
1.Gold L. S., De Roos A. J. & Waters M. et al., "Systematic Literature Review of Uses and Levels of Occupational Exposure to Tetrachloroethylene," Journal of occupational and environmental hygiene, Vol.5, No.12(2008), pp.807-839.
2.Shi Wenjing and Zhu Yuliang: Research on tetrachloroethylene as a substitute reagent for carbon tetrachloride, resource conservation and environmental protection, 2015, No. 08, pp. 60-62.
3.Fang Li, Yuan Xiangqian, song Hongyu: Study on the reaction of oxychlorination of 1,2-dichloroethane to tetrachloroethylene, natural gas chemical industry (C1 chemistry and chemical industry), 2017, No. 02, pp. 70-75.
You may like
Related articles And Qustion
See also
Lastest Price from Tetrachloroethylene manufacturers

US $10.00/kg2025-04-21
- CAS:
- 127-18-4
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100
US $0.00/kg2025-04-15
- CAS:
- 127-18-4
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons