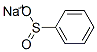Benzenesulfinic acid sodium salt: applications in organic synthesis and safety
General Description
Benzenesulfinic acid sodium salt is a versatile compound widely used in organic synthesis. It serves as a valuable sulfur source for generating thioethers through direct C-H functionalization, providing an environmentally friendly alternative to traditional methods. As a traceless linker in solid-phase synthesis, it enables efficient production of diverse organic compounds. Additionally, its role as a key reagent in copper(II)-catalyzed reactions facilitates the construction of complex organic molecules, particularly valuable intermediates for pharmaceuticals and agrochemicals. However, strict safety measures must be observed during handling and storage due to its potential hazards. Overall, this compound plays a crucial role in advancing synthetic methodologies and the production of valuable chemical compounds.

Figure 1. Benzenesulfinic acid sodium salt
Applications in organic synthesis
Sulfur source
Benzenesulfinic acid sodium salt serves as a valuable sulfur source in the generation of thioethers through direct C-H functionalization. This compound has found application in a novel sulfenylation method facilitated by ammonium iodide, allowing for the synthesis of regioselective derivatives of flavones, indole, and arylimidazo[1,2-a]pyridines in good yields. Unlike traditional methods utilizing iodine, this approach offers an environmentally friendly and odorless alternative, thereby enhancing current thioether-producing techniques. The stability and lack of odor in Benzenesulfinic acid sodium salt make it particularly attractive for such synthetic processes. By exploiting the reactivity of this compound along with the unique properties of ammonium iodide, this method represents a significant advancement in the field of sulfur-based synthesis, showcasing the potential for innovative and sustainable sulfur incorporation in organic molecules. 1
Traceless linker
Benzenesulfinic acid sodium salt, as a traceless linker, has found diverse applications in solid-phase synthesis. It has been successfully employed in the synthesis of pyrazolines, isoxazolines, 3,4-dihydropyrimidine-2-ones, and 1,2,4,5-tetrasubstituted imidazoles, thiazoles, and oxazoles. In the solid-phase synthetic procedure for pyrazolines and isoxazolines, key steps include sulfinate S-alkylation, sulfone anion alkylation, gamma-hydroxy sulfone to gamma-ketosulfone oxidation, and traceless product release via elimination-cyclization. This method yielded a library of 12 pyrazolines and isoxazolines. For the synthesis of 3,4-dihydropyrimidine-2-ones, the procedure involves sulfinate acidification, condensation of urea or thiourea with aldehydes and sulfinic acid, and traceless product release via a one-pot cyclization-dehydration process, resulting in the synthesis of 18 compounds. Furthermore, in the preparation of 1,2,4,5-tetrasubstituted imidazoles, thiazoles, and oxazoles, the traceless solid-phase sulfone linker strategy involves sulfinate acidification, sulfinic acid condensation with aldehyde and amine, and traceless product release by a one-pot elimination-cyclization reaction, leading to the synthesis of another library of 18 compounds. Overall, the use of benzenesulfinic acid sodium salt as a traceless linker has enabled efficient and versatile solid-phase synthesis of diverse organic compounds, demonstrating its wide applicability in synthetic chemistry. 2,3,4
Construction of complex organic molecules
Benzenesulfinic acid sodium salt is a versatile chemical compound with various applications in organic synthesis. In the study "Copper(II)-Catalyzed Domino Synthesis of 4-Benzenesulfonyl Isoxazoles from 2-Nitro-1,3-enynes, Amines, and Sodium Benzenesulfinate," its application as a key reagent is highlighted. This compound participates in a three-component reaction under the catalysis of copper(II), leading to the formation of fully substituted 4-benzenesulfonyl isoxazoles. The mild reaction conditions and high chemoselectivity make this method an attractive choice for organic synthesis. Benzenesulfinic acid sodium salt plays a crucial role in this reaction by providing the benzenesulfonyl group, which is incorporated into the final product. The resulting 4-benzenesulfonyl isoxazoles are valuable intermediates in the synthesis of pharmaceuticals, agrochemicals, and functional materials. This work demonstrates the significance of Benzenesulfinic acid sodium salt as a building block in the construction of complex organic molecules, showcasing its potential impact on the development of new synthetic methodologies and the production of valuable chemical compounds. 5
Safety
Benzenesulfinic acid sodium salt is a white crystalline powder with a variety of industrial applications. In terms of safety, it is important to handle this compound with care. Firstly, exposure should be minimized, and strict personal protective equipment should be worn when handling the substance, including gloves, goggles, and a lab coat. Additionally, sodium benzenesulfinate should be stored in a cool, dry, well-ventilated area, away from incompatible materials. In case of ingestion, inhalation, or contact with skin or eyes, immediate medical attention is necessary. Furthermore, proper disposal procedures should be followed to prevent environmental contamination. Overall, strict adherence to safety protocols is crucial when working with sodium benzenesulfinate to ensure the well-being of individuals and the environment. 6
Reference
1. Ding Y, Wu W, Zhao W, Li Y, Xie P, Huang Y, Liu Y, Zhou A. Generation of thioethers via direct C-H functionalization with sodium benzenesulfinate as a sulfur source. Org Biomol Chem. 2016 Jan 28;14(4):1428-1431.
2. Li W, Lam Y. A facile solid-phase synthesis of 1,2,4,5-tetrasubstituted imidazoles using sodium benzenesulfinate as a traceless linker. J Comb Chem. 2005 Sep-Oct;7(5):644-647.
3. Li W, Lam Y. Solid-phase synthesis of 3,4-dihydro-1H-pyrimidine-2-ones using sodium benzenesulfinate as a traceless linker. J Comb Chem. 2005 Sep-Oct;7(5):721-725.
4. Chen Y, Lam Y, Lai YH. Solid-phase synthesis of pyrazolines and isoxazolines with sodium benzenesulfinate as a traceless linker. Org Lett. 2003 Apr 3;5(7):1067-1069.
5. Ge J, Ding Q, Long X, Liu X, Peng Y. Copper(II)-Catalyzed Domino Synthesis of 4-Benzenesulfonyl Isoxazoles from 2-Nitro-1,3-enynes, Amines, and Sodium Benzenesulfinate. J Org Chem. 2020 Nov 6;85(21):13886-13894.
6. Sodium benzenesulfinate. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases, CAS Number: 873-55-2.
Related articles And Qustion
Lastest Price from BENZENESULFINIC ACID SODIUM SALT manufacturers

US $0.00/KG2025-04-21
- CAS:
- 873-55-2
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/kg2025-04-21
- CAS:
- 873-55-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt