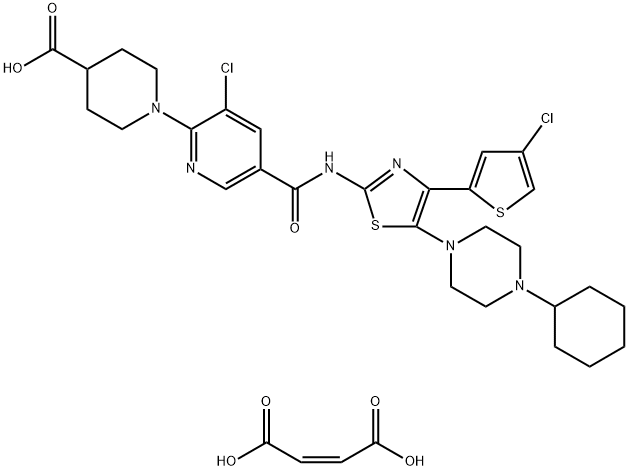
Avatrombopag maleate: Preparation, Appication, Bioactivity
General description
Avatrombopag maleate is a thrombopoietin receptor (TPO) agonist developed by AkaRx Inc and approved by the US FDA in May 2018 as Doptelet (20mg). For the treatment of low platelet count (thrombocytopenia CIT) in adult patients with chronic liver disease (CLD) who are scheduled to undergo medical or dental surgery. In April 2020, Avatopopal maleate tablets were approved by the China Medical Products Administration under the trade name Sukexin for the treatment of adult patients with thrombocytopenia associated with chronic liver disease who have elective diagnostic operations or surgeries. Thrombocytopenia is one of the common complications in patients with chronic liver disease. Previously, only platelet infusion was approved for the treatment of CLD-related thrombocytopenia in China. As the first therapeutic drug for CLD-related thrombocytopenia in China, Avatopopal maleate tablet was launched in China to fill the domestic drug gap in this field. It has introduced the world's leading new treatment scheme of "strong, durable, safe and convenient" for Chinese patients with CLD related thrombocytopenia.
Preparation
After the inclusion of Avatrombopag maleate with cyclodextrin and commovidone, Avatrombopag maleate loses its original crystallinity and enters the gap of the inclusion compound in a molecular state. The drug has a high dispersion, and the inclusion material contains a number of hydrophilic alcohol hydroxyl groups, which makes the inclusion compound have good wettability, thus achieving the solubility of the drug. Greatly increased drug solubility, molecular drugs easily through the biofilm, thus improving drug bioavailability. The invention through a large number of screening tests found that in the commonly used cyclodextrin types, hydroxypropyl β cyclodextrin and Avatrombopag maleate inclusion effect is relatively good, especially when the molar ratio of Avatrombopag maleate and added hydroxypropyl β cyclodextrin is 1:2.4, the inclusion effect of the drug is the best. However, even if the inclusion compound of Avatrombopag maleate prepared at the optimal ratio, the inclusion efficiency was still low, which was equivalent to the inclusion of about 4.8 g of cyclodextrin per gram of drug based on the optimal molar ratio of 1:2.4. The volume or mass of the inclusion compound was too large, which was not favorable for the further preparation of the inclusion compound drug. Moreover, the stability of the inclusion compound is poor. At the same time, due to the large amount of cyclodextrin in the inclusion process, the preparation cost is also high, which is not conducive to the daily production and preparation. In the further study accidentally found, when in the preparation of hydroxypropyl β cyclodextrin inclusion compound added a certain amount of copolymer vidone, especially the copolymer vidone for hydroxypropyl-β-cyclodextrin quality of 3.0 ~ 7. 0%, cyclodextrin inclusion efficiency significantly improved, can make the use of hydroxypropyl β cyclodextrin reduce about half the amount, The solubilization effect and stabilization effect of Avatrombopag were the best [1].
Application
Polymorphic forms of Avatrombopag maleate
Preparation of (I) comprises either (a) dissolving avatrombopag maleate in a mixture of organic solvent or organic solvent and water at elevated temperature, (b) cooling the solution to room temperature, and (c) isolating the amorphous avatrombopag maleate, or (a1) dissolving avatrombopag maleate in organic solvent, (b1) adding an antisolvent, and (c1) isolating the amorphous avatrombopag maleate. Preferred Process: In the process (P1) the elevated temperature is at 60-95 degrees C. In the process (P1) the amorphous form comprises isolated by lyophilization or spray-drying and filtration. In the process (P2) the crystalline avatrombopag maleate is isolated by filtration. In the process (P2) the elevated temperature is at 60-95 degrees C. In the process (P3) the elevated temperature is at 60-95 degrees C. In the process (P3) the maleate is isolated by filtration. Preferred Components: The maleate has PXRD pattern as shown in figure-10 of the specification. In the process (P1) the organic solvent comprises 1,4-dioxane, tetrahydrofuran, or anisole and dimethyl sulfoxide. The organic solvent is dimethyl sulfoxide and antisolvent is water. The form M1 further has PXRD pattern having significant peaks at 2 theta angles of 11.48, 14.55, 17.67, 18.42, 18.85, 19.41 , 22.00, 23.55, 23.87 and 24.69 plus minus 0.2 degrees , and the M1 has PXRD pattern as shown in figure-6 of the specification. In the process (P2) the antisolvent comprises water or methyl tertiary-butyl ether. The maleate form M3 further has PXRD pattern having significant peaks at 2 theta angles of 4.94, 8.47, 9.75, 15.91 , 16.07,17.95, 21 .74, 24.10 and 24.30 plus minus 0.2 degrees and the PXRD pattern as shown in figure-8 of the specification. In the process (P3) the antisolvent comprises isobutyl acetate, ethyl acetate, anisole, methyl isobutyl ketone, 2-butanol, 2-propanol or 2- methyl-1 -propanol [2].
Preparation of impurity of avatrombopag maleate
The preparation of the compound (I) involves dissolving dimethylamine in an organic solvent to prepare a dimethylamine solution with a concentration of 0.5-1.5 mol/L, preferably 1 mol/L, dissolving the compound (II) in the organic solvent and performing reflux reaction with dimethylamine solution for 6-10 hours. Preferred Components: The organic solvent is dichloromethane, methanol (preferred), ethanol (preferred), ethyl acetate, acetone, tetrahydrofuran (preferred) or 1,4-dioxane. Preferred Properties: The molar ratio of compound (II) to dimethylamine is 1:8-15, preferably 1:11.1. The mass-volume ratio of compound (II) to the organic solvent is 1:8-20 g/ml, preferably 1:10 g/ml [3].
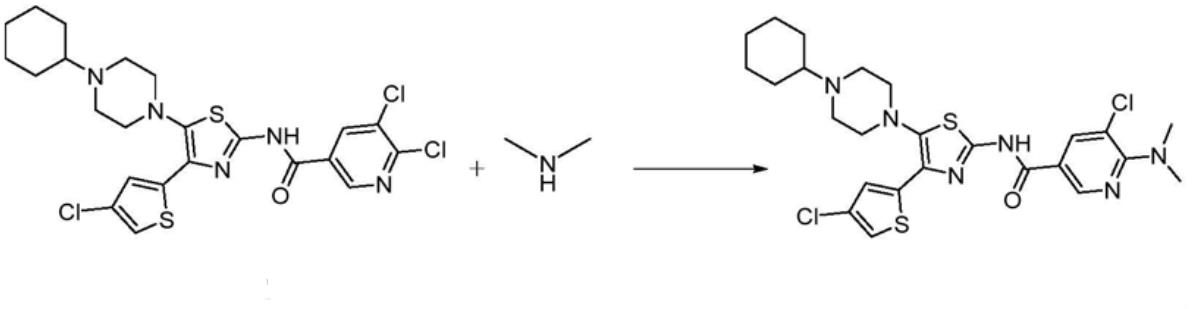
Fig. 1 The synthetic method of impurity avatrombopag maleate impurity.
Bioactivity
Thrombopoietin Receptor Agonist Treatment of Thrombocytopenia
Avatrombopog moleote (E-5501) is a novel, orally active thrombopoietin receptor (TPO-R) agonist that mimics the biological effects of TPO. In preclinical studies, avatrombopag maleate stimulated megakaryocyte colony growth in a dose-dependent fashion, with maximum activity similar to that of TPO. A synergistic effect between avatrombopag maleate and TPO was also observed. Avatrombopag maleate induced platelet production in normal subjects in a dose-related manner. The drug was well tolerated at doses required to induce platelet production in normal individuals. The efficacy and safety of avatrombopag maleate have been investigated in patients with chronic immune thrombocytopenia (ITP) who had received at least one prior treatment for ITP. In a dose-finding study there were statistically more platelet responses at day 28 with avatrombopag maleate 20 mg/day than for placebo or avatrombopag maleate 2.5 mg/day. A 6-month extension study showed that the drug is able to support prolonged platelet responses. In these studies, avatrombopag maleate was in general well tolerated. Further development of avatrombopag maleate as a potential new treatment for thrombocytopenia is warranted [4].
The treatment of periprocedural thrombocytopenia in patients with chronic liver disease
Avatrombopag maleate (Doptelet; Dova Pharmaceuticals) is an oral, small-molecule second-generation thrombopoietin (TPO) receptor agonist under development for the treatment of thrombocytopenia. Recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of periprocedural thrombocytopenia in patient
You may like
Related articles And Qustion
See also
Lastest Price from Avatrombopag maleate manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 677007-74-8
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 20kg/Month

US $0.00/g2025-01-13
- CAS:
- 677007-74-8
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 50kg/Month