Astaxanthin: Biosynthesis, applications and production status
General description
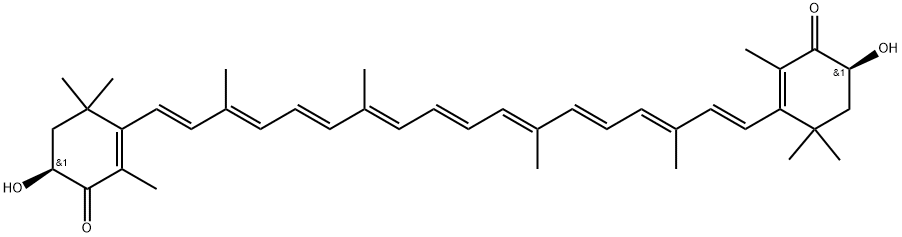
Astaxanthin is a keto-carotenoid.[1] It belongs to a larger class of chemical compounds known as terpenes (as a tetraterpenoid) built from five carbon precursors, isopentenyl diphosphate, and dimethylallyl diphosphate. Astaxanthin is classified as a xanthophyll (originally derived from a word meaning "yellow leaves" since yellow plant leaf pigments were the first recognized of the xanthophyll family of carotenoids), but currently employed to describe carotenoid compounds that have oxygen-containing components, hydroxyl (-OH) or ketone (C=O), such as zeaxanthin and canthaxanthin. Indeed, astaxanthin is a metabolite of zeaxanthin and/or canthaxanthin, containing both hydroxyl and ketone functional groups. Like many carotenoids, astaxanthin is a lipid-soluble pigment. Its red-orange colour is due to the extended chain of conjugated (alternating double and single) double bonds at the centre of the compound. This chain of conjugated double bonds is also responsible for the antioxidant function of astaxanthin (as well as other carotenoids) as it results in a region of decentralized electrons that can be donated to reduce a reactive oxidizing molecule. Its appearance is as follows:

Figure 1 Appearance of Astaxanthin.
Biosynthesis
Astaxanthin biosynthesis starts with three molecules of isopentenyl pyrophosphate (IPP) and one molecule of dimethylallyl pyrophosphate (DMAPP) that are combined by IPP isomerase and converted to geranylgeranyl pyrophosphate (GGPP) by GGPP synthase. Two molecules of GGPP are then coupled by phytoene synthase to form phytoene. Next, phytoene desaturase creates four double bonds in the phytoene molecule to form lycopene. After desaturation, lycopene cyclase first forms γ-carotene by converting one of the ψ acyclic ends of the lycopene as a β-ring, then subsequently converts the other to form β-carotene. From β-carotene, hydrolases (blue) are responsible for the inclusion of two 3-hydroxy groups, and ketolases (green) for the addition of two 4-keto groups, forming multiple intermediate molecules until the final molecule, astaxanthin, is obtained.[2]
Applications
Astaxanthin is used as a dietary supplement and feed supplement as food colorant for salmon, crabs, shrimp, chickens and egg production.[3] The primary use of synthetic astaxanthin today is as an animal feed additive to impart coloration, including farm-raised salmon and chicken egg yolks. Synthetic carotenoid pigments colored yellow, red or orange represent about 15–25% of the cost of production of commercial salmon feed. Today, almost all commercial astaxanthin for aquaculture is produced synthetically. The primary human application for astaxanthin is as a dietary supplement, although as of 2018, there was insufficient evidence from medical research to show that it affects disease risk or health of people, and it remains under preliminary research. In 2018, the European Food Safety Authority sought scientific information from manufacturers of dietary supplements about the safety of astaxanthin.
Production status
The cost of astaxanthin production, high market price and lack of a leading fermentation production systems, combined with the intricacies of chemical synthesis mean that research into alternative fermentation production methods has been carried out. Metabolic engineering offers the opportunity to create biological systems for the production of a specific target compound. The metabolic engineering of bacteria (Escherichia coli) recently allowed production of astaxanthin at >90% of the total carotenoids, providing the first engineered production system capable of efficient astaxanthin production.[4] Astaxanthin biosynthesis proceeds from beta-carotene via either zeaxanthin or canthaxanthin. Historically, it has been assumed that astaxanthin biosynthesis proceeds along both routes. However, recent work has suggested that efficient biosynthesis may, in fact, proceed from beta-carotene to astaxanthin via zeaxanthin. The production of astaxanthin by metabolic engineering, in isolation, will not provide a suitable alternative to current industrial methods. Rather, a bioprocess approach should be adopted. Such an approach would consider fermentation conditions and economics, as well as downstream processing (extraction). Carotenoid extraction has been studied extensively, for example, the extraction of canthaxanthin (a precursor to astaxanthin) was studied within an E. coli production process demonstrating that extraction efficiency was increased substantially when two solvents, acetone and methanol, were used sequentially rather than as a combined solution.
References
[1]Margalith, P. Z. (1999). "Production of ketocarotenoids by microalgae". Applied Microbiology and Biotechnology. 51 (4): 431–8. doi:10.1007/s002530051413. PMID 10341427.
[2]Barredo, Jose; García-Estrada, Carlos; Kosalkova, Katarina; Barreiro, Carlos (2017-07-30). "Biosynthesis of Astaxanthin as a Main Carotenoid in the Heterobasidiomycetous Yeast Xanthophyllomyces dendrorhous". Journal of Fungi. 3 (3): 44. doi:10.3390/jof3030044. ISSN 2309-608X. PMC 5715937. PMID 29371561.
[3]Ambati, Ranga Rao; Phang, Siew-Moi; Ravi, Sarada; Aswathanarayana, Ravishankar Gokare (2014-01-07). "Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review". Marine Drugs. 12 (1): 128–152. doi:10.3390/md12010128. PMC 3917265. PMID 24402174.
[4]Scaife, M. A.; Burja, A. M.; Wright, P. C. (2009). "Characterization of cyanobacterial β-carotene ketolase and hydroxylase genes inEscherichia coli, and their application for astaxanthin biosynthesis". Biotechnology and Bioengineering. 103 (5): 944–955. doi:10.1002/bit.22330. PMID 19365869.
You may like
Related articles And Qustion
See also
Lastest Price from Astaxanthin manufacturers

US $100.00-80.00/Kg2025-11-18
- CAS:
- 472-61-7
- Min. Order:
- 1Kg
- Purity:
- 1%-10%
- Supply Ability:
- tons

US $0.00/kg2025-09-22
- CAS:
- 472-61-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg