Applications of 2,2,2-Trifluoroethanol
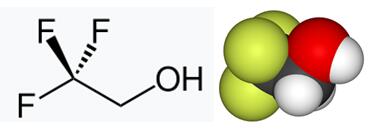
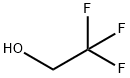
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol. Thus, 2,2,2-Trifluoroethanol forms stable complexes also with heterocycles (e.g. THF or pyridine) through hydrogen bonding.2,2,2-Trifluoroethanol is a non-aqueous co-solvent that serves as tool to study protein folding. 2,2,2-Trifluoroethanol is also used in various pharmaceutical, chemical and engineering applications.
2,2,2-Trichloroethanol is an organic compound related to ethanol, except the hydrogen atoms at position 2 are replaced with chlorine atoms. In humans, its pharmacological effects are similar to those of its prodrugs, chloral hydrate and chlorobutanol. It has, historically, been used as a sedative hypnotic. The hypnotic drug triclofos (2,2,2-trichloroethyl phosphate) is metabolized in vivo to 2,2,2-trichloroethanol. Chronic exposure may result in kidney and liver damage. 2,2,2-Trichloroethanol can be added to SDS-PAGE gels in order to enable fluorescent detection of proteins without a staining step. This imaging step is compatible with later analysis by, e.g., immunoblotting.
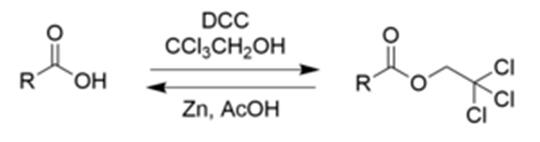
2,2,2-trichloroethanol is an effective protecting group for carboxylic acids due to its ease in addition and removal.
2,2,2 trifluoroethanol is produced industrially by hydrogenation or the hydride reduction of derivatives of trifluoroacetic acid, such as the esters or acid chloride[1].
2,2,2 trifluoroethanol can also be prepared by hydrogenolysis of compounds of generic formula CF3−CHOH−OR (where R is hydrogen or an alkyl group containing from one to eight carbon atoms), in the presence of a palladium containing catalyst deposited on activated charcoal. As a co-catalyst for this conversion tertiary aliphatic amines like triethylamine are commonly employed.
2,2,2 trifluoroethanol is used as a solvent in organic chemistry[2,3]. Oxidations of sulfur compounds using hydrogen peroxide are effectively conducted in 2,2,2 trifluoroethanol[4]. 2,2,2 trifluoroethanol can also be used as a protein denaturant. In biology Trifluoroethanol is used as a co-solvent in protein folding studies with NMR spectroscopy: this solvent can effectively solubilize both peptides and proteins. Depending upon its concentration, 2,2,2 trifluoroethanol can strongly affect the three-dimensional structure of proteins.
Industrially 2,2,2 trifluoroethanol is employed as a solvent for nylon as well as in applications of the pharmaceutical field.
2,2,2 trifluoroethanol is a key precursor for the inhaled anaesthetic isoflurane, listed on the World Health Organization's List of Essential Medicines.
Trifluoroethanol, specifically 2,2,2 trifluoroethanol is also used in biochemistry as an inhibitor to study enzymes. 2,2,2 trifluoroethanol competitively inhibits alcohol dehydrogenase for example[5].
Oxidation of 2,2,2 trifluoroethanol yields trifluoroacetaldehyde or trifluoroacetic acid. It also serves as a source of the trifluoromethyl group for various chemical reactions (Still-Gennari modification of HWE reaction). 2,2,2-trifluoro-1-vinyloxyethane, an inhaled drug introduced clinically under the tradename Fluoromar, features a vinyl ether of 2,2,2 trifluoroethanol. This species was prepared by the reaction of 2,2,2 trifluoroethanol with acetylene.
2,2,2 trifluoroethanol is classified as toxic to blood, the reproductive system, bladder, brain, upper respiratory tract and eyes. Research has shown 2,2,2 trifluoroethanol to be a testicular toxicant in rats and dogs.
References
[1] Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, 2007.
[2] Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B. (2004). "Fluorinated Alcohols: A New Medium for Selective and Clean Reaction". Synlett (Review) (1): 18–29.
[3] Shuklov, Ivan A.; Dubrovina, Natalia V.; Börner, Armin (2007). "Fluorinated Alcohols as Solvents, Cosolvents and Additives in Homogeneous Catalysis". Synthesis (Review). 2007 (19): 2925–2943.
[4] Kabayadi S. Ravikumar; Venkitasamy Kesavan; Benoit Crousse; Danièle Bonnet-Delpon; Jean-Pierre Bégué (2003). "Mild and Selective Oxidation of Sulfur Compounds in Trifluorethanol: Diphenyl Disulfide and Methyle Phenyl Sulfoxide". Organic Syntheses. 80: 184.
[5] Taber, Richard L. (1998). "Wiley Online Library". Biochemical Education. 26 (3): 239–242.
See also
Lastest Price from 2,2,2-Trifluoroethanol manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 75-89-8
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $5.00-2.00/KG2024-10-11
- CAS:
- 75-89-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg