Application research of Ethyl 4,4,4-trifluoroacetoacetate
Introduction
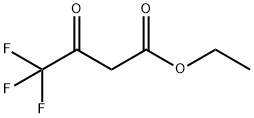
Ethyl 4,4,4-trifluoroacetoacetate (Figure 1) is a new pharmaceutical and plant protection intermediate synthesized in China in 2003. It is the main raw material for the synthesis of pesticide thiazopyr. Its mechanism of action is to produce fluoroacetyl coenzyme A after metabolism, and form fluorocitric acid with oxaloacetic acid, blocking the cycle of tricarboxylic acid and destroying energy synthesis.[1]

Synthesis of Ethyl 4,4,4-Trifluoroacetoacetate
Ethyl 4,4,4-trifluoroacetoacetate was synthesized from ethyl acetate and 4,4,4-trifluoroacetate (mole ratio3:1) with sodium ethoxide. The reaction was carried at 50 ºC for 5 hours, ethanol from the reaction was removed by distilling the azeotrope of ethyl acetate and ethanol to complete the reaction. The yield was 75.9%.[2]
Application research example
1. Biginelli Reaction Using Ethyl 4,4,4-trifluoroacetoacetate
A series of 5-ethoxycarbonyl-6-trifluoromethyl-6-hydroxyl-3,4,5,6-tetrahydropyrimidin -2(1H)-one derivatives was synthesized by Biginelli reaction with Ethyl 4,4,4-trifluoroacetoacetate,substituted benzaldehydes and urea(or thiourea)as raw materials,in the place of sulfamic acid as catalyst and ethanol as solvent,the yield can gain 28.1%-96.1%. This method has mild reaction conditions,simple operation and environmental friendliness.[3]
2. Asymmetric reduction of ethyl 4,4,4-trifluoroacetoacetate
In this study, whole cells of Saccharomyces uvarum SW-58 were applied in an aqueous-organic solvent biphasic system for the asymmetric reduction of ethyl 4,4,4-trifluoroacetoacetate to ethyl (R)-4,4,4-trifluoro-3-hydroxybutanoate [(R)-2]. The results of reduction in different aqueous-organic solvent biphasic systems showed that dibutylphthalate provided the best compromise between the biocompatibility and the partition of substrate and product among the solvents tested. To optimize the reaction, several factors such as reaction pH, temperature, shaking speed, volume ratio of the aqueous phase to the organic phase and ratio of biomass/substrate were investigated. It was found that the change of these factors obviously influenced the conversion and initial reaction rate, and had a minor effect on the enatiomeric excess of the product. Under the optimal conditions, 85.0% of conversion and 85.2% of enatiomeric excess were achieved. The bioconversion in the biphasic system was more efficient compared with that in the monophasic aqueous system, and product concentration as high as 54.6 g/L was reached in the organic phase without addition of co-enzyme.[4]
3.Synthetic studies of Isomeric pyrazolopyrimidines
During our studies into preparing analogues of pyrazolopyrimidine as ATP synthesis inhibitors of Mycobacterium tuberculosis, a regiospecific condensation reaction between ethyl 4,4,4-trifluoroacetoacetate and 3-(4-fluorophenyl)-1H-pyrazol-5-amine was observed which was dependent on the specific reaction conditions employed. This work identifies optimized reaction conditions to access either the pyrazolo[3,4-β]pyridine or the pyrazolo[1,5-α]pyrimidine scaffold. This has led to the structural confirmation of the previously reported pyrazolopyrimidine 17b which was reported as pyrazolo[1,5-α]pyrimidine structure 2 which was corrected to pyrazolo[3,4-β]-pyrimidine 19.[5]
4.New fluorinated dihydropyranonaphthoquinone compounds synthesis
This study presents the synthesis of new fluorinated dihydropyranonapthoquinone compounds by means of microwave-prompted four-component reactions using lawson, fluorinated aromatic aldehydes, ethyl 4,4,4-trifluoroacetoacetate and ammonium acetate. The products were thoroughly characterized by spectroscopic methods and assessed for their anti-inflammatory activity in lipopolysaccharide-stimulated RAW264.7 macrophage cells. All synthesized compounds demonstrated notable inhibitory effects on NO production with IC50 ranging from 1.67 ± 0.02 to 28.81 ± 2.44 μM without causing significant toxicity. Interestingly, compound 8n showed the most potent NO inhibitory activity in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells with IC50 value of 1.67 ± 0.02 μM. Moreover, compounds 8c,h,i,l significantly decreased the levels of pro-inflammatory cytokines IL-1β and IL-6 in LPS-stimulated RAW264.7 macrophages. These results indicate the potential of these compounds as promising candidates for the development of new anti-inflammatory agents.[6]
5.Novel 1,2,3-triazole/isoxazole functionalized 2H-Chromene derivatives synthesis
A series of novel 2-(1,2,3-triazolylmethoxy) 5a-q and isoxazole tagged 6a-g 2H-Chromene derivatives were prepared starting from salicylaldehyde and ethyl-4,4,4-trifluoroacetoacetate via cyclization to form ethyl 2-hydroxy-2-(trifluoromethyl)-2H-Chromene-3-carboxylate 3. Compound 3 on reaction with propargyl bromide resulted compound 4 and was independently reacted with aryl/alkyl azides and aryl aldoximes obtained 2-(1,2,3-triazolylmethoxy) and isoxazole tagged 2H-Chromene derivatives 5a-q, 6a-i, respectively. Compounds 6 were further hydrolysed to acid derivatives 7a-g. All the products 5a-q, 6a-i, 7a-g were screened for cytotoxic activity against four human cancer cell lines and among all the compounds, 5f, 5g, 5l, 5q showed promising activity at <20 μM concentration.[7]
References
1. Yang Xh,et al.Study on subacute oral toxicity of ethyl 4,4,4-trifluoroacetoacetate in rats[J].Chinese J Ind Med ,2013,26(04):271-273.
2. Zhang Gh, et al. Synthesis of Ethyl 4,4,4-Trifluoroacetoacetate [J].Agrochemicals,2007,(12):823-824.DOI:10.16820/j.cnki.1006-0413.2007.12.009.
3. Li Gc,et al.Biginelli Reaction Using Ethyl 4,4,4-Trifluoroacetoacetate[J].Anhui chemical industry,2025,51(03):88-90.
4. He J, Mao X, Sun Z, Zheng P, Ni Y, Xu Y. Microbial synthesis of ethyl (R)-4,4,4-trifluoro-3-hydroxybutanoate by asymmetric reduction of ethyl 4,4,4-trifluoroacetoacetate in an aqueous-organic solvent biphasic system. Biotechnol J. 2007;2(2):260-265. doi:10.1002/biot.200600106
5. Choi PJ, Lu GL, Sutherland HS, et al. Synthetic studies towards isomeric pyrazolopyrimidines as potential ATP synthesis inhibitors of Mycobacterium tuberculosis. Structural correction of reported N-(6-(2-(dimethylamino)ethoxy)-5-fluoropyridin-3-yl)-2-(4-fluorophenyl)-5-(trifluoromethyl)pyrazolo[1,5-α]pyrimidin-7-amine. Tetrahedron Lett. 2022;90:None. doi:10.1016/j.tetlet.2021.153611
6. Nguyen Thi QG, Nguyen HT, Dang Thi TA, et al. Improved synthesis and anti-inflammatory activity of new fluorinated dihydropyranonaphthoquinone compounds. Bioorg Med Chem Lett. Published online October 11, 2025. doi:10.1016/j.bmcl.2025.130434
7. Ratnakar Reddy K, Sambasiva Rao P, Jitender Dev G, et al. Synthesis of novel 1,2,3-triazole/isoxazole functionalized 2H-Chromene derivatives and their cytotoxic activity. Bioorg Med Chem Lett. 2014;24(7):1661-1663. doi:10.1016/j.bmcl.2014.02.069
You may like
Lastest Price from Ethyl 4,4,4-trifluoroacetoacetate manufacturers

US $10.00/KG2025-06-30
- CAS:
- 372-31-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 372-31-6
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons