Application research of Chlorhexidine diacetate
Introduction
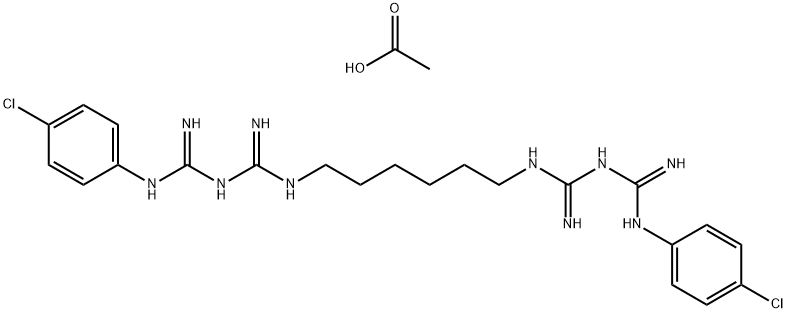
Chlorhexidine diacetate (Figure 1) has a broad efficacy against microorganisms, and is successfully used as an oral antimicrobial and antiplaque agent. Chlorhexidine has proven antifungal action against C. albicans and the advantage of being effective even against strains that are resistant to other local antifungal agents available to treat this infection in the oral cavity. The chlorhexidine has been successfully used in Dentistry and is available in different formulations,including digluconate, hydrochloride, and diacetate formulations. The first formulation is most commonly used in mouthwashes because it is more soluble in water, while the last two formulations are more soluble in ethanol. The use of chlorhexidine is considered the gold standard antiseptic treatment for this agent has strong bactericidal and bacteriostatic capacities.[1]

Cytotoxicity and teratogenicity of chlorhexidine diacetate
Intra-uterine contraceptive devices are associated with an increased incidence of pelvic infections, possible due to the introduction of vaginal bacteria into the uterus at insertion. One potential means to overcome this problem is the use of a device which releases the antimicrobial agent chlorhexidine although such an approach carries with it the risk of adverse effects on the endometrium and, possibly, teratogenic effects. Cultured monolayers of endometrial cells were used to assess the cytotoxicity of both chlorhexidine and chlorhexidine-releasing devices. The results indicated that the agent is toxic at concentrations of 1 microg mL(-1) and that the devices potentiated the toxicity. When the devices were tested in a guinea-pig model, endometrial damage was seen only at the high dose of chlorhexidine, suggesting that there is greater distribution of chlorhexidine in-vivo. Assessment of the teratogenic effects of chlorhexidine in rat embryonic limb bud tissue cells in-vitro showed that the foetal cells were highly susceptible to the toxic effects of chlorhexidine, but that there was no evidence of teratogenicity. Overall, the findings suggest that chlorhexidine-releasing devices may be a safe means of reducing infections related to intra-uterine devices, but that the chlorhexidine may have a toxic effect on foetal cells.[1]
Chlorhexidine diacetate versus chlorhexidine free base dressings
The aim of this study was to investigate the in vitro antimicrobial performance of a chlorhexidine diacetate dressing and a chlorhexidine free base dressing to determine if the free base form of chlorhexidine has the potential to be an effective alternative to the chlorhexidine salts used in conventional, chlorhexidine-based antimicrobial dressings. Dressing samples were inoculated with clinically relevant pathogenic microorganisms including Gram-positive and Gram-negative bacteria, yeasts and fungus, and subsequently evaluated for in vitro log 10 reduction at 1-, 3-, and 7-day time points. Chlorhexidine mole content was also calculated as a function of dressing surface area for both sample types to allow for formulation-independent comparison between the dressings. The chlorhexidine free base dressing demonstrated >0.5 log 10 superior mean antimicrobial efficacy at 67% of the experimental time points and equivalent mean antimicrobial efficacy (≤0.5 log 10 different) at the remaining time points when compared with the chlorhexidine diacetate dressing. The chlorhexidine free base dressing was also found to contain 36% less chlorhexidine mole content than the chlorhexidine diacetate dressing. The results suggest that a dressing formulated with chlorhexidine free base can deliver in vitro antimicrobial performance at both a magnitude and rate that meets or exceeds that of a chlorhexidine diacetate-based dressing, while also allowing for a reduction in total chlorhexidine content per dressing. These findings could be of particular interest to researchers developing new antimicrobial technologies as well as to infection preventionists when evaluating antimicrobial products for use on clinical patients at elevated risk of infection.[2]
Combined use nitric oxide with chlorhexidine diacetate
The presence of bacteria and biofilm on medical device surfaces has been linked to serious infections, increased health care costs, and failure of medical devices. Therefore, antimicrobial biointerfaces and medical devices that can thwart microbial attachment and biofilm formation are urgently needed. Both nitric oxide (NO) and chlorhexidine diacetate (CHXD) possess broad-spectrum antibacterial properties. In the past, individual polymer release systems of chlorhexidine diacetate and NO donor S-nitroso-N-acetylpenicillamine (SNAP) incorporated polymer platforms have attracted considerable attention for biomedical/therapeutic applications. However, the combination of the two surfaces has not yet been explored. Herein, the synergy of NO and chlorhexidine diacetate was evaluated to create an antimicrobial medical-grade silicone rubber. The 10 wt% SNAP films were fabricated using solvent casting with a topcoat of chlorhexidine diacetate (1, 3, and 5 wt%) to generate a dual-active antibacterial interface. Chemiluminescence studies confirmed the NO release from SNAP-CHXD films at physiologically relevant levels (0.5-4 × 10-10 mol min-1 cm-2 ) for at least 3 weeks and chlorhexidine diacetate release for at least 7 days. Further characterization of the films via SEM-EDS confirmed uniform distribution of SNAP and presence of chlorhexidine diacetate within the polymer films without substantial morphological changes, as confirmed by contact angle hysteresis. Moreover, the dual-active SNAP-CHXD films were able to significantly reduce Escherichia coli and Staphylococcus aureus bacteria (>3-log reduction) compared to controls with no explicit toxicity towards mouse fibroblast cells. The synergy between the two potent antimicrobial agents will help combat bacterial contamination on biointerfaces and enhance the longevity of medical devices.[3]
Incorporation of chlorhexidine diacetate in provisional cements
To test the antibacterial capacities and tensile strengths of three commercially available provisional cements to which chlorhexidine diacetate was added and compare them to the same unmodified cements. Sixty cylindrical samples were prepared from either three noneugenol provisional cements or the same cements modified by the addition of chlorhexidine diacetate at 7.5% w/w, with a total of 360 samples. The cements tested included Tempbond NE, Rely X Temp NE and Freegenol. Forty-eight samples from each cement were aged in saline that was replaced twice a week for up to 96 days. Twelve of these samples were removed at either 1, 15, 30 or 96 days and assessed for antibacterial properties against Streptococcus mutans with an agar diffusion test. Twelve samples of each cement, with and without chlorhexidine diacetate, were also tested 7 days after the initial setting for their tensile strength using a diametrical tensile strength test applied with an Instron machine. The results were analysed using either one-way or three-way anova. The addition of chlorhexidine diacetate resulted in provisional cements with antibacterial properties that persisted through ageing in saline for up to 96 days. The addition of chlorhexidine did not reduce the diametrical strength of the cements.The addition of chlorhexidine diacetate to provisional cements rendered all three cements antibacterial against S. mutans and this activity was maintained even after prolonged ageing of the cements, without compromising their tensile strength at 7 days.[4]
References
1. Maluf CV, Peroni LV, Menezes LR, Coutinho W, Lourenço EJV, Telles DM. Evaluation of the physical and antifungal effects of chlorhexidine diacetate incorporated into polymethyl methacrylate. J Appl Oral Sci. 2020;28:e20190039. Published 2020 Jan 10. doi:10.1590/1678-7757-2019-0039
2. Holinga GJ, McGuire JE. In vitro antimicrobial effects of chlorhexidine diacetate versus chlorhexidine free base dressings. J Wound Care. 2020;29(Sup5a):S22-S28. doi:10.12968/jowc.2020.29.Sup5a.S22
3. Chug MK, Massoumi H, Wu Y, Brisbois EJ. Prevention of medical device infections via multi-action nitric oxide and chlorhexidine diacetate releasing medical grade silicone biointerfaces. J Biomed Mater Res A. 2022;110(6):1263-1277. doi:10.1002/jbm.a.37372
Lewinstein I, Zenziper E, Block J, Kfir A. Incorporation of chlorhexidine diacetate in provisional cements: antimicrobial activity against Streptococcus mutans and the effect on tensile strength in vitro. Int Endod J. 2012;45(11):1010-1017. doi:10.1111/j.1365-2591.2012.02063.x
You may like
See also
Lastest Price from Chlorhexidine diacetate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 206986-79-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 206986-79-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month